Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging
- PMID: 29896378
- PMCID: PMC5961444
- DOI: 10.1039/c8sc01153b
Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging
Abstract
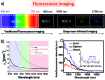
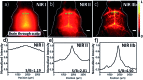
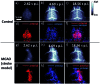
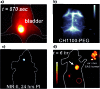
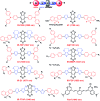
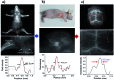
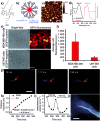
In recent years, owing to unsatisfactory clinical imaging clarity and depths in the living body for early diagnosis and prognosis, novel imaging modalities with high bioimaging performance have been actively explored. The remarkable headway made in the second near-infrared region (NIR-II, 1000-1700 nm) has promoted the development of biomedical imaging significantly. NIR-II fluorescence imaging possesses a number of merits which prevail over the traditional and NIR-I (400-900 nm) imaging modalities in fundamental research, such as reduced photon scattering, as well as auto-fluorescence and improved penetration depth. Functional probes for instant and precise feedback of in vivo information are at the core of this modality for superb imaging. Herein, we review the recently developed fluorophores including carbon nanotubes, organic small molecules, quantum dots, conjugated polymers and rare-earth-doped materials to present superior and multifunctionality of biomedical imaging in the NIR-II regions (1000-1700 nm).
Figures

References
-
- Weissleder R. Science. 2006;312:1168–1171. - PubMed
-
- Hoffman J. M., Gambhir S. S. Radiology. 2007;244:39–47. - PubMed
-
- Chen H., Dong B. L., Tang Y. H., Lin W. Y. Acc. Chem. Res. 2017;50:1410–1422. - PubMed
-
- Hong G. S., Antaris A. L., Dai H. J. Nat. Biomed. Eng. 2017;1:0010.
-
- Hou J. T., Ren W. X., Li K., Seo J., Shama A., Yu X. Q., Kim J. S. Chem. Soc. Rev. 2017;53:2076–2090. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

