9,10-Azaboraphenanthrene-containing small molecules and conjugated polymers: synthesis and their application in chemodosimeters for the ratiometric detection of fluoride ions
- PMID: 29896385
- PMCID: PMC5956975
- DOI: 10.1039/c8sc00688a
9,10-Azaboraphenanthrene-containing small molecules and conjugated polymers: synthesis and their application in chemodosimeters for the ratiometric detection of fluoride ions
Abstract
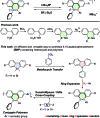
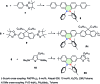
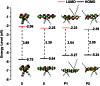
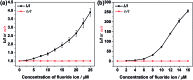
The introduction of main group elements into conjugated scaffolds is emerging as a key route to novel optoelectronic materials. Herein, an efficient and versatile way to synthesize polymerizable 9,10-azaboraphenanthrene (BNP)-containing monomers by aromaticity-driven ring expansion reactions between highly antiaromatic borafluorene and azides is reported, and the corresponding conjugated small molecules and polymers are developed as well. The BNP-containing small molecules and conjugated polymers showed good air/moisture stability and notable fluorescence properties. Addition of fluoride ions to the BNP-based small molecules and polymers induced a rapid change in the emission color from blue to green/yellow, respectively, accompanied by strong intensity changes. The conjugated polymers showed better ratiometric sensing performance than small molecules due to the exciton migration along the conjugated chains. Further experiments showed that the sensing process is fully reversible. The films prepared by solution-deposition of BNP-based compounds in the presence of polycaprolactone also showed good ratiometric sensing for fluoride ions.
Figures




Similar articles
-
Simple conjugated polymers with on-chain phosphorescent iridium(III) complexes: toward ratiometric chemodosimeters for detecting trace amounts of mercury(II).Chemistry. 2010 Oct 25;16(40):12158-67. doi: 10.1002/chem.201000748. Chemistry. 2010. PMID: 20839372
-
Cationic Conjugated Polyelectrolytes with Aggregation-Induced Ratiometric Fluorescence.Macromol Rapid Commun. 2022 Sep;43(18):e2100899. doi: 10.1002/marc.202100899. Epub 2022 Mar 16. Macromol Rapid Commun. 2022. PMID: 35247010
-
Azulene-Based π-Functional Materials: Design, Synthesis, and Applications.Acc Chem Res. 2021 Apr 6;54(7):1737-1753. doi: 10.1021/acs.accounts.0c00893. Epub 2021 Mar 11. Acc Chem Res. 2021. PMID: 33691401
-
Water-Soluble Conjugated Organic Molecules as Optical and Electrochemical Materials for Interdisciplinary Biological Applications.Acc Chem Res. 2019 Nov 19;52(11):3211-3222. doi: 10.1021/acs.accounts.9b00427. Epub 2019 Oct 14. Acc Chem Res. 2019. PMID: 31609571 Review.
-
How to Design Donor-Acceptor Based Heterocyclic Conjugated Polymers for Applications from Organic Electronics to Sensors.Top Curr Chem (Cham). 2019 Apr 22;377(3):12. doi: 10.1007/s41061-019-0237-4. Top Curr Chem (Cham). 2019. PMID: 31011839 Review.
Cited by
-
peri-Acenoacene Ribbons with Zigzag BN-Doped Peripheries.J Am Chem Soc. 2022 Nov 30;144(47):21470-21484. doi: 10.1021/jacs.2c06803. Epub 2022 Nov 17. J Am Chem Soc. 2022. PMID: 36394460 Free PMC article.
-
A pillar[5]arene-based planar chiral charge-transfer dye with enhanced circularly polarized luminescence and multiple responsive chiroptical changes.Chem Sci. 2023 Jan 3;14(4):987-993. doi: 10.1039/d2sc06000k. eCollection 2023 Jan 25. Chem Sci. 2023. PMID: 36755718 Free PMC article.
-
Enhanced N-directed electrophilic C-H borylation generates BN-[5]- and [6]helicenes with improved photophysical properties.Chem Sci. 2022 Jan 4;13(4):1136-1145. doi: 10.1039/d1sc06513k. eCollection 2022 Jan 26. Chem Sci. 2022. PMID: 35211280 Free PMC article.
-
Avenue to novel o-carboranyl boron compounds - reactivity study of o-carborane-fused aminoborirane towards organic azides.Chem Sci. 2024 Feb 22;15(13):4839-4845. doi: 10.1039/d4sc00489b. eCollection 2024 Mar 27. Chem Sci. 2024. PMID: 38550674 Free PMC article.
-
(Hetero)arene-fused boroles: a broad spectrum of applications.Chem Sci. 2020 Nov 24;12(1):128-147. doi: 10.1039/d0sc05676f. Chem Sci. 2020. PMID: 34163585 Free PMC article. Review.
References
-
- Gessner B. D., Beller M., Middaugh J. P., Whitford G. M. N. Engl. J. Med. 1994;330:95–99. - PubMed
-
- Singh P., Barjatiya M., Dhing S., Bhatnagar R., Kothari S., Dhar V. Urol. Res. 2001;29:238–244. - PubMed
-
- Zheng X., Zhu W., Liu D., Ai H., Huang Y., Lu Z. ACS Appl. Mater. Interfaces. 2014;6:7996–8000. - PubMed
-
- Gale P. A. Acc. Chem. Res. 2006;39:465–475. - PubMed
-
- Gale P. A., Caltagirone C. Chem. Soc. Rev. 2015;44:4212–4227. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

