On-demand release of Candida albicans biofilms from urinary catheters by mechanical surface deformation
- PMID: 29897277
- PMCID: PMC6276112
- DOI: 10.1080/08927014.2018.1474461
On-demand release of Candida albicans biofilms from urinary catheters by mechanical surface deformation
Abstract
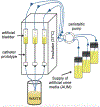
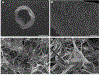
Candida albicans is a leading cause of catheter-associated urinary tract infections and elimination of these biofilm-based infections without antifungal agents would constitute a significant medical advance. A novel urinary catheter prototype that utilizes on-demand surface deformation is effective at eliminating bacterial biofilms and here the broader applicability of this prototype to remove fungal biofilms has been demonstrated. C. albicans biofilms were debonded from prototypes by selectively inflating four additional intralumens surrounding the main lumen of the catheters to provide the necessary surface strain to remove the adhered biofilm. Deformable catheters eliminated significantly more biofilm than the controls (>90% eliminated vs 10% control; p < 0.001). Mechanical testing revealed that fungal biofilms have an elastic modulus of 45 ± 6.7 kPa with a fracture energy of 0.4-2 J m-2. This study underscores the potential of mechanical disruption as a materials design strategy to combat fungal device-associated infections.
Keywords: Biofilm; Candida albicans; catheter-associated infection; elastic modulus; mechanical deformation; urinary catheter.
Figures



References
-
- Baier RE, Shafrin EG, Zisman WA. 1968. Adhesion: mechanisms that assist or impede it. Science. 162:1360–1368. - PubMed
-
- Bol M, Ehret AE, Bolea Albero A, Hellriegel J, Krull R. 2013. Recent advances in mechanical characterisation of biofilm and their significance for material modelling. Crit Rev Biotechnol. 33:145–171. - PubMed
-
- Bonhomme J, d’Enfert C. 2013. Candida albicans biofilms: building a heterogeneous, drug-tolerant environment. Curr Opin Microbiol. 16:398–403. - PubMed
-
- Burnier M, Rutschmann B, Nussberger J, Versaggi J, Shahinfar S, Waeber B, Brunner HR. 1993. Salt-dependent renal effects of an angiotensin II antagonist in healthy subjects. Hypertension. 22:339–347. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
