Regulation of Skeletal Homeostasis
- PMID: 29897433
- PMCID: PMC6173473
- DOI: 10.1210/er.2018-00050
Regulation of Skeletal Homeostasis
Abstract
Landmark advances in skeletal biology have arisen mainly from the identification of disease-causing mutations and the advent of rapid and selective gene-targeting technologies to phenocopy human disease in mice. Here, we discuss work on newly identified mechanisms controlling the remodeling of bone, communication of bone cells with cells of other lineages, and crosstalk between bone and vital organs as these relate to the therapeutic targeting of the skeleton.
Figures


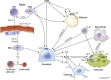
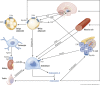
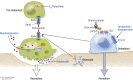
References
-
- Evans CH. John Hunter and the origins of modern orthopaedic research. J Orthop Res. 2007;25(4):556–560. - PubMed
-
- Kölliker A. Die normale Resorption des Knochengewebes und ihre Bedeutung für die Entsehung der typischen Knoehenformen. Leipzig: Vogel; 1873.
-
- Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. - PubMed
-
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37(4):411–417. - PubMed
-
- Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12(12):2014–2023. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

