The c-Abl inhibitor, Radotinib HCl, is neuroprotective in a preclinical Parkinson's disease mouse model
- PMID: 29897434
- PMCID: PMC6005030
- DOI: 10.1093/hmg/ddy143
The c-Abl inhibitor, Radotinib HCl, is neuroprotective in a preclinical Parkinson's disease mouse model
Abstract
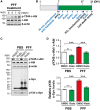
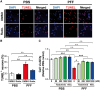
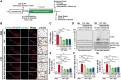
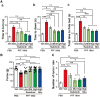
Accumulating evidence suggests that the non-receptor tyrosine kinase c-Abl plays an important role in the progression of Parkinson's disease (PD) and c-Abl inhibition could be neuroprotective in PD and related α-synucleinopathies. Nilotinib, a c-Abl inhibitor, has shown improved motor and cognitive symptoms in PD patients. However, issues concerning blood-brain barrier (BBB) penetration, lack of selectivity and safety still remain. Radotinib HCl is a selective Bcr-Abl kinase inhibitor that not only effectively access the brain, but also exhibits greater pharmacokinetic properties and safety profiles compared to Nilotinib and other c-Abl inhibitors. Here, we show the neuroprotective efficacy of Radotinib HCl, a brain penetrant c-Abl inhibitor, in a pre-clinical model of PD. Importantly, in vitro studies demonstrate that the treatment of Radotinib HCl protects the α-synuclein preformed fibrils (PFF)-induced neuronal toxicity, reduces the α-synuclein PFF-induced Lewy bodies (LB)/Lewy neurites (LN)-like pathology and inhibits the α-synuclein PFF-induced c-Abl activation in primary cortical neurons. Furthermore, administration of Radotinib HCl inhibits c-Abl activation and prevents dopaminergic neuron loss, neuroinflammation and behavioral deficits following α-synuclein PFF-induced toxicity in vivo. Taken together, our findings indicate that Radotinib HCl has beneficial neuroprotective effects in PD and provides an evidence that selective and brain permeable c-Abl inhibitors can be potential therapeutic agents for the treatment of PD and related α-synucleinopathies.
Figures



References
-
- Lang A.E., Lozano A.M. (1998) Parkinson's disease. First of two parts. N. Engl. J. Med., 339, 1044–1053. - PubMed
-
- Lang A.E., Lozano A.M. (1998) Parkinson's disease. Second of two parts. N. Engl. J. Med., 339, 1130–1143. - PubMed
-
- Dawson T.M., Dawson V.L. (2003) Molecular pathways of neurodegeneration in Parkinson's disease. Science, 302, 819–822. - PubMed
-
- Tsang A.H., Chung K.K. (2009) Oxidative and nitrosative stress in Parkinson's disease. Biochim. Biophys. Acta, 1792, 643–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

