The Tat protein transport system: intriguing questions and conundrums
- PMID: 29897510
- PMCID: PMC5995166
- DOI: 10.1093/femsle/fny123
The Tat protein transport system: intriguing questions and conundrums
Abstract
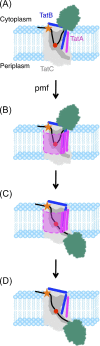
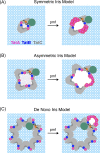
The Tat machinery catalyzes the transport of folded proteins across the cytoplasmic membrane in bacteria and the thylakoid membrane in plants. Transport occurs only in the presence of an electric field (Δψ) and/or a pH (ΔpH) gradient, and thus, Tat transport is considered to be dependent on the proton motive force (pmf). This presents a fundamental and major challenge, namely, that the Tat system catalyzes the movement of large folded protein cargos across a membrane without collapse of ion gradients. Current models argue that the active translocon assembles de novo for each cargo transported, thus providing an effective gating mechanism to minimize ion leakage. A limited structural understanding of the intermediates occurring during transport and the role of the pmf in stabilizing and/or driving this process have hindered the development of more detailed models. A fundamental question that remains unanswered is whether the pmf is actually 'consumed', providing an energetic driving force for transport, or alternatively, whether its presence is instead necessary to provide the appropriate environment for the translocon components to become active. Including addressing this issue in greater detail, we explore a series of additional questions that challenge current models, and, hopefully, motivate future work.
Figures

References
-
- Alami M, Luke I, Deitermann S et al. . Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell 2003;12:937–46. - PubMed
-
- Alder NN, Theg SM. Energetics of protein transport across biological membranes. a study of the thylakoid DeltapH-dependent/cpTat pathway Cell 2003;112:231–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

