Dose-Related Severity Sequence, and Risk-Based Integration, of Chemically Induced Health Effects
- PMID: 29897534
- PMCID: PMC6190798
- DOI: 10.1093/toxsci/kfy124
Dose-Related Severity Sequence, and Risk-Based Integration, of Chemically Induced Health Effects
Erratum in
-
Corrigendum to "Dose-Related Severity Sequence, and Risk-Based Integration, of Chemically Induced Health Effects".Toxicol Sci. 2019 Jul 1;170(1):248. doi: 10.1093/toxsci/kfz105. Toxicol Sci. 2019. PMID: 31046096 Free PMC article. No abstract available.
Abstract
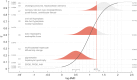
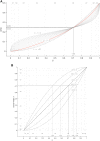
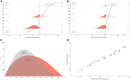
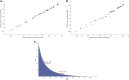
Risk assessment of chemical hazards is typically based on single critical health effects. This work aims to expand the current approach by characterizing the dose-related sequence of the development of multiple (lower- to higher-order) toxicological health effects caused by a chemical. To this end a "reference point profile" is defined as the relation between benchmark doses for considered health effects, and a standardized severity score determined for these effects. For a given dose of a chemical or mixture the probability for exceeding the reference point profile, thereby provoking lower- to higher-order effects, can be assessed. The overall impact at the same dose can also be derived by integrating contributions across all health effects following severity-weighting. In its generalized form the new impact metric relates to the probability of response for the most severe health effects. Reference points (points of departure) corresponding to defined levels of response can also be estimated. The proposed concept, which is evaluated for dioxin-like chemicals, provides an alternative for characterizing the low-dose region below the reference point for a severe effect like cancer. The shape and variability of the reference point profile add new dimensions to risk assessment, which for example extends the characterization of chemical potency, and the concept of acceptable effect sizes for individual health effects. Based on the present data the method shows high stability at low doses/responses, and is also robust to differences in severity categorization of effects. In conclusion, the novel method proposed enables risk-based integration of multiple dose-related health effects. It provides a first step towards a more comprehensive characterization of chemical toxicity, and suggests a potential for improved low-dose risk assessment.
Figures


References
-
- Andersen M. E., Krewski D. (2010). The vision of toxicity testing in the 21st century: Moving from discussion to action. Toxicol. Sci. 117, 17–24. - PubMed
-
- Ankley G. T., Bennett R. S., Erickson R. J., Hoff D. J., Hornung M. W., Johnson R. D., Mount D. R., Nichols J. W., Russom C. L., Schmieder P. K., et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. - PubMed
-
- Becker R. A., Patlewicz G., Simon T. W., Rowlands J. C., Budinsky R. A. (2015). The adverse outcome pathway for rodent liver tumor promotion by sustained activation of the aryl hydrocarbon receptor. Reg. Toxicol. Pharmacol. 73, 172–190. - PubMed
-
- Bogdanffy M. S., Daston G., Faustman E. M., Kimmel C. A., Kimmel G. L., Seed J., Vu V. (2001). Harmonization of cancer and noncancer risk assessment: Proceedings of a consensus-building workshop. Toxicol. Sci. 61, 18–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

