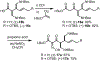A Nitrone Dipolar Cycloaddition Strategy toward an Enantioselective Synthesis of Massadine
- PMID: 29897770
- PMCID: PMC6674976
- DOI: 10.1021/acs.orglett.8b01464
A Nitrone Dipolar Cycloaddition Strategy toward an Enantioselective Synthesis of Massadine
Abstract
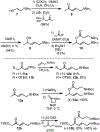
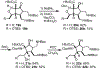
An enantioselective route to the C,D-bicycle of massadine is reported. Enantiopure intermediates were generated by a single stereoselective reduction using the Corey-Bakshi-Shibata reagent. This initial stereoinduction was translated into the five contiguous stereocenters of the massadine D-ring by a synthetic route that features a diastereoselective and stereospecific Ireland-Claisen rearrangement of a trianionic enolate followed by a diastereoselective nitrone dipolar cycloaddition of a highly electron-poor oxime.
Conflict of interest statement
The author declares no competing financial interest.
Figures
References
-
- Köck M; Grube A; Seiple IB; Baran PS Angew. Chem., Int. Ed 2007, 46, 6586–6594. - PubMed
-
- Arndt H-D; Riedrich M Angew. Chem., Int. Ed 2008, 47, 4785–4788. - PubMed
-
- Jin Z Nat. Prod. Rep 2011, 28, 1143–1191. - PubMed
-
- Lindel T Chemistry and Biology of the Pyrrole–Imidazole Alkaloids. Alkaloids Chem. Biol 2017, 77, 117–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources