Macrophage Rac2 Is Required to Reduce the Severity of Cigarette Smoke-induced Pneumonia
- PMID: 29897791
- PMCID: PMC6290940
- DOI: 10.1164/rccm.201712-2388OC
Macrophage Rac2 Is Required to Reduce the Severity of Cigarette Smoke-induced Pneumonia
Abstract
Rationale: Cigarette smoking is prevalent in the United States and is the leading cause of preventable diseases. A prominent complication of smoking is an increase in lower respiratory tract infections (LRTIs). Although LRTIs are known to be increased in subjects that smoke, the mechanism(s) by which this occurs is poorly understood.
Objectives: Determine how cigarette smoke (CS) reduces reactive oxygen species (ROS) production by the phagocytic NOX2 (NADPH oxidase 2), which is essential for innate immunity in lung macrophages.
Methods: NOX2-derived ROS and Rac2 (Ras-related C3 botulinum toxin substrate 2) activity were determined in BAL cells from wild-type and Rac2-/- mice exposed to CS or cadmium and in BAL cells from subjects that smoke. Host defense to respiratory pathogens was analyzed in mice infected with Streptococcus pneumoniae.
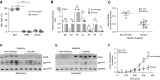
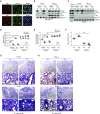
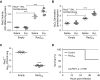
Measurements and main results: NOX2-derived ROS in BAL cells was reduced in mice exposed to CS via inhibition of the small GTPase Rac2. These mice had greater bacterial burden and increased mortality compared with air-exposed mice. BAL fluid from CS-exposed mice had increased levels of cadmium, which mediated the effect on Rac2. Similar observations were seen in human subjects that smoke. To support the importance of Rac2 in the macrophage immune response, overexpression of constitutively active Rac2 by lentiviral administration increased NOX2-derived ROS, decreased bacterial burden in lung tissue, and increased survival compared with CS-exposed control mice.
Conclusions: These observations suggest that therapies to maintain Rac2 activity in lung macrophages restore host defense against respiratory pathogens and diminish the prevalence of LRTIs in subjects that smoke.
Keywords: NADPH oxidase 2; heavy metals; innate immunity; respiratory tract infections.
Figures





Comment in
-
C-rac-king the Code of Smoke-induced Pneumonia Susceptibility.Am J Respir Crit Care Med. 2018 Nov 15;198(10):1246-1248. doi: 10.1164/rccm.201807-1217ED. Am J Respir Crit Care Med. 2018. PMID: 30063845 Free PMC article. No abstract available.
-
Reply to Eapen et al.: Dysfunctional Immunity and Microbial Adhesion Molecules in Smoking-induced Pneumonia.Am J Respir Crit Care Med. 2019 Jan 15;199(2):251-252. doi: 10.1164/rccm.201809-1727LE. Am J Respir Crit Care Med. 2019. PMID: 30290120 Free PMC article. No abstract available.
-
Dysfunctional Immunity and Microbial Adhesion Molecules in Smoking-induced Pneumonia.Am J Respir Crit Care Med. 2019 Jan 15;199(2):250-251. doi: 10.1164/rccm.201808-1553LE. Am J Respir Crit Care Med. 2019. PMID: 30290125 No abstract available.
References
-
- U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention; 2014.
-
- Garmendia J, Morey P, Bengoechea JA. Impact of cigarette smoke exposure on host–bacterial pathogen interactions. Eur Respir J. 2012;39:467–477. - PubMed
-
- Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. Active Bacterial Core Surveillance Team. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681–689. - PubMed
-
- Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL114439/HL/NHLBI NIH HHS/United States
- R56 ES027464/ES/NIEHS NIH HHS/United States
- R01 ES029981/ES/NIEHS NIH HHS/United States
- K08 HL123940/HL/NHLBI NIH HHS/United States
- R01 HL128502/HL/NHLBI NIH HHS/United States
- R01 HL126596/HL/NHLBI NIH HHS/United States
- R35 HL135710/HL/NHLBI NIH HHS/United States
- R01 ES015981/ES/NIEHS NIH HHS/United States
- R01 HL077783/HL/NHLBI NIH HHS/United States
- P30 NS047466/NS/NINDS NIH HHS/United States
- R01 HL110950/HL/NHLBI NIH HHS/United States
- R01 AI118805/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

