Clinical trials in neonates: How to optimise informed consent and decision making? A European Delphi survey of parent representatives and clinicians
- PMID: 29897934
- PMCID: PMC5999079
- DOI: 10.1371/journal.pone.0198097
Clinical trials in neonates: How to optimise informed consent and decision making? A European Delphi survey of parent representatives and clinicians
Abstract
Objectives: Parental consent for the participation of their neonate in neonatal research is influenced by the quality of the information delivered and the interaction between parents and investigators. Failure to provide important information may lead to difficulties in the decision making process of parents. This Delphi survey aims to establish a consensus between parent representatives of neonatal associations and healthcare professionals concerning the information deemed essential by both parties in order to improve the recruitment of neonates into clinical trials.
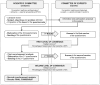
Method: This study was conducted in Europe among parent representatives and healthcare professionals. In this 3-phase study, 96 items were defined by the Scientific Committee (CS), composed of 11 clinicians (from 8 countries) and 1 parent representative of the European network of neonatal associations. Then the Committee of Experts (CE) composed of 16 clinicians were matched by country with 16 national parent representatives and evaluated these items in two rounds. The importance of each item was evaluated by each member of the CE on a scale between 1 and 9 based on their personal experience.
Results: Fifty eight items reached the second and final level of consensus. In contrast to clinicians, parent representatives preferred to be informed about the study by the physician in charge of their child. They also favoured additional support during the informed consent process and stated that both parents need to agree and sign.
Conclusion: The set of 58 items on which parents and clinicians reached consensus will be helpful to healthcare professionals seeking parental consent for the inclusion of a neonate in a clinical trial. Providing parents with information about the trial by the investigator in the presence of the patient's neonatologist, developing closer contacts with parents and informing them of the available support by parents associations may be helpful for parents.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Olski TM, Lampus SF, Gherarducci G, Saint Raymond A. Three years of paediatric regulation in the European Union. Eur J Clin Pharmacol. 2011;67: 245–252. doi: 10.1007/s00228-011-0997-4 - DOI - PubMed
-
- Chappuy H, Doz F, Blanche S, Gentet J-C, Pons G, Tréluyer J-M. Parental consent in paediatric clinical research. Arch Dis Child. 2006;91: 112–116. doi: 10.1136/adc.2005.076141 - DOI - PMC - PubMed
-
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1982. 1997;45: 1337–1355. - PubMed
-
- Snowdon C, Elbourne D, Garcia J. Declining enrolment in a clinical trial and injurious misconceptions: is there a flipside to the therapeutic misconception? Clin Ethics. 2007;2: 193–200. doi: 10.1258/147775007783560193 - DOI
-
- Tait AR, Voepel-Lewis T, Robinson A, Malviya S. Priorities for disclosure of the elements of informed consent for research: a comparison between parents and investigators. Paediatr Anaesth. 2002;12: 332–336. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical