Recombinant purified buffalo leukemia inhibitory factor plays an inhibitory role in cell growth
- PMID: 29897967
- PMCID: PMC5999108
- DOI: 10.1371/journal.pone.0198523
Recombinant purified buffalo leukemia inhibitory factor plays an inhibitory role in cell growth
Abstract
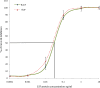
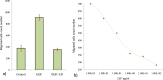
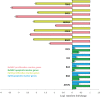
Leukemia Inhibitory Factor (LIF) is a polyfunctional cytokine, involved in numerous regulatory effects in vivo and in vitro, varying from cell proliferation to differentiation, and has therapeutic potential for treating various diseases. In the current study, a COS-1 cell line overexpressing recombinant Buffalo LIF (rBuLIF) was established. The rBuLIF was purified to homogeneity from the total cell lysate of COS-1 cells using a two-step affinity chromatography. The purified LIF was confirmed by western blot and mass spectrometer (MS/MS). Particularly, high-resolution MS has identified the rBuLIF with 73% of sequence coverage with highest confidence parameters and with the search engine score of 4580. We determined the molecular weight of rBuLIF protein to be 58.99 kDa and 48.9 kDa with and without glycosylation, respectively. Moreover, the purified rBuLIF was verified to be functionally active by measuring the growth inhibition of M1 myeloid leukemia cells, revealing a maximum inhibition at 72 hours and half-maximal effective concentration (EC50) of 0.0555 ng/ml, corresponding to a specific activity of >1.6×10(7) units/mg. Next, we evaluated the effect of rBuLIF on buffalo mammary epithelial cell lines for its role in involution and also identified the IC50 value for BuMEC migrating cells to be 77.8 ng/ml. Additionally, the treatment of MECs (BuMEC and EpH4) displayed significant (P < 0.05) reduction in growth progression, as confirmed by qRT-PCR analysis, suggesting its strong involvement in the involution of the mammary gland in vivo. Thus, we conclude that the glycosylated rBuLIF, purified from COS-1 cells was found to be functionally active as its natural counterpart.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Taga T. gp130 and the interleukin-6 family cytokines. Annu. Rev. Immunol. 1997, 15:797–819. doi: 10.1146/annurev.immunol.15.1.797 - DOI - PubMed
-
- Tomida M, Yamamoto-Yamaguchi Y, Hozumi M. Characterization of a factor inducing differentiation of mouse myeloid leukemic cells purified from conditioned medium of mouse Ehrlich ascites tumor cells. FEBS Lett. 1984, 178:291–6. - PubMed
-
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988, 336:688–690. doi: 10.1038/336688a0 - DOI - PubMed
-
- Smith A. Embryonic Stem Cells In Stem Cell Biology, Marshak D.R., Gardner R.L., and Gottlieb D., eds. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; ), 2001, pp. 205–230.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

