Reduction in terminally differentiated T cells in virologically controlled HIV-infected aging patients on long-term antiretroviral therapy
- PMID: 29897981
- PMCID: PMC5999291
- DOI: 10.1371/journal.pone.0199101
Reduction in terminally differentiated T cells in virologically controlled HIV-infected aging patients on long-term antiretroviral therapy
Abstract
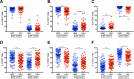
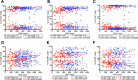
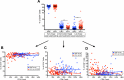
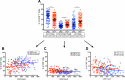
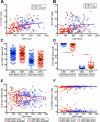
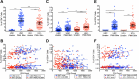
Several studies have shown an increased accumulation of terminally differentiated T cells during HIV infection, suggestive of exhaustion/senescence, causing dysregulation of T cell homeostasis and function and rapid HIV disease progression. We have investigated whether long-term antiretroviral therapy (ART), which controls viremia and restores CD4 T cell counts, is correlated with reduction in terminally differentiated T cells, improved ratios of naïve to memory and function of T cells in 100 virologically controlled HIV-infected patients. We show that while the median frequencies of terminally differentiated CD4+ and CD8+ T cells (CD28-, CD27-, CD57+ and CD28-CD57+), were higher in the virologically controlled HIV-infected patients' cohort compared with uninfected individuals' cohort, the frequencies of these cells significantly decreased with increasing CD4 T cell counts in HIV-infected patients. Although, the naïve CD4+ and CD8+ T cells were lower in HIV patients' cohort than uninfected cohort, there was a significant increase in both naïve CD4+ and CD8+ T cells with increasing CD4 T cell counts in HIV-infected patients. The underlying mechanism behind this increased naïve CD4+ and CD8+ T cells in HIV-infected patients was due to an increase in recent thymic emigrants, CD4+CD31+, as compared to CD4+CD31-. The CD4+ T cells of HIV-infected patients produced cytokines, including IL-2, IL-10 and IFN-γ comparable to uninfected individuals. In conclusion, virologically controlled HIV-infected patients on long-term ART show a significant reduction in terminally differentiated T cells, suggestive of decreased exhaustion/senescence, and improvement in the ratios of naïve to memory and function of T cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging.PLoS One. 2014 Feb 27;9(2):e89444. doi: 10.1371/journal.pone.0089444. eCollection 2014. PLoS One. 2014. PMID: 24586783 Free PMC article.
-
Immunosenescent CD57+CD4+ T-cells accumulate and contribute to interferon-γ responses in HIV patients responding stably to ART.Dis Markers. 2011;31(6):337-42. doi: 10.3233/DMA-2011-0847. Dis Markers. 2011. PMID: 22182806 Free PMC article.
-
Altered T cell differentiation associated with loss of CD27 and CD28 in HIV infected Indian individuals.Cytometry B Clin Cytom. 2012 Jan;82(1):43-53. doi: 10.1002/cyto.b.20610. Epub 2011 Jun 21. Cytometry B Clin Cytom. 2012. PMID: 21695776
-
Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era.J Int AIDS Soc. 2016 Mar 3;19(1):20697. doi: 10.7448/IAS.19.1.20697. eCollection 2016. J Int AIDS Soc. 2016. PMID: 26945343 Free PMC article. Review.
-
T cell regeneration in HIV-infected subjects under highly active antiretroviral therapy (review).Int J Mol Med. 1999 Jul;4(1):91-7. doi: 10.3892/ijmm.4.1.91. Int J Mol Med. 1999. PMID: 10373643 Review.
Cited by
-
Current Status of Latency Reversing Agents Facing the Heterogeneity of HIV-1 Cellular and Tissue Reservoirs.Front Microbiol. 2020 Jan 24;10:3060. doi: 10.3389/fmicb.2019.03060. eCollection 2019. Front Microbiol. 2020. PMID: 32038533 Free PMC article. Review.
-
HIV Infection-Associated Frailty: The Solution for Now Is Antiretroviral Drugs: A Perspective.J Int Assoc Provid AIDS Care. 2019 Jan-Dec;18:2325958219831045. doi: 10.1177/2325958219831045. J Int Assoc Provid AIDS Care. 2019. PMID: 30803299 Free PMC article.
-
Evaluation of HIV-specific T-cell responses in HIV-infected older patients with controlled viremia on long-term antiretroviral therapy.PLoS One. 2020 Sep 17;15(9):e0236320. doi: 10.1371/journal.pone.0236320. eCollection 2020. PLoS One. 2020. PMID: 32941433 Free PMC article.
-
Plasma Levels of Secreted Cytokines in Virologically Controlled HIV-Infected Aging Adult Individuals on Long-Term Antiretroviral Therapy.Viral Immunol. 2024 May;37(4):202-215. doi: 10.1089/vim.2023.0123. Viral Immunol. 2024. PMID: 38717822 Free PMC article.
-
Inversed Ratio of CD39/CD73 Expression on γδ T Cells in HIV Versus Healthy Controls Correlates With Immune Activation and Disease Progression.Front Immunol. 2022 Apr 22;13:867167. doi: 10.3389/fimmu.2022.867167. eCollection 2022. Front Immunol. 2022. PMID: 35529864 Free PMC article.
References
-
- Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50(2):137–47. doi: 10.1097/QAI.0b013e3181926c28 . - DOI - PMC - PubMed
-
- Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833–42. Epub 2013/10/24. doi: 10.1093/gerona/glt168 ; PubMed Central PMCID: PMCPMC4067117. - DOI - PMC - PubMed
-
- Appay V, Sauce D. Assessing immune aging in HIV-infected patients. Virulence. 2017;8(5):529–38. Epub 2016/06/16. doi: 10.1080/21505594.2016.1195536 ; PubMed Central PMCID: PMCPMC5538339. - DOI - PMC - PubMed
-
- Nikolich-Zugich J. T cell aging: naive but not young. J Exp Med. 2005;201(6):837–40. doi: 10.1084/jem.20050341 . - DOI - PMC - PubMed
-
- Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002;23(12):580–5. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

