CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets
- PMID: 29898400
- PMCID: PMC6581793
- DOI: 10.1016/j.celrep.2018.05.035
CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets
Abstract
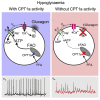
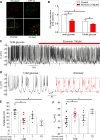
Glucagon, the principal hyperglycemic hormone, is secreted from pancreatic islet α cells as part of the counter-regulatory response to hypoglycemia. Hence, secretory output from α cells is under high demand in conditions of low glucose supply. Many tissues oxidize fat as an alternate energy substrate. Here, we show that glucagon secretion in low glucose conditions is maintained by fatty acid metabolism in both mouse and human islets, and that inhibiting this metabolic pathway profoundly decreases glucagon output by depolarizing α cell membrane potential and decreasing action potential amplitude. We demonstrate, by using experimental and computational approaches, that this is not mediated by the KATP channel, but instead due to reduced operation of the Na+-K+ pump. These data suggest that counter-regulatory secretion of glucagon is driven by fatty acid metabolism, and that the Na+-K+ pump is an important ATP-dependent regulator of α cell function.
Keywords: Ca2+; KATP; fasting; glucose; islet; liver; metabolism.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes.Cell Metab. 2013 Dec 3;18(6):871-82. doi: 10.1016/j.cmet.2013.10.014. Cell Metab. 2013. PMID: 24315372 Free PMC article.
-
Pancreatic expression of CPT1A is essential for whole body glucose homeostasis by supporting glucose-stimulated insulin secretion.J Biol Chem. 2025 Feb;301(2):108187. doi: 10.1016/j.jbc.2025.108187. Epub 2025 Jan 13. J Biol Chem. 2025. PMID: 39814231 Free PMC article.
-
ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1-/- mouse alpha-cells.Diabetes. 2004 Dec;53 Suppl 3:S181-9. doi: 10.2337/diabetes.53.suppl_3.s181. Diabetes. 2004. PMID: 15561909
-
'Resistance is futile?' - paradoxical inhibitory effects of KATP channel closure in glucagon-secreting α-cells.J Physiol. 2020 Nov;598(21):4765-4780. doi: 10.1113/JP279775. Epub 2020 Aug 7. J Physiol. 2020. PMID: 32716554 Free PMC article. Review.
-
Regulatory Role of Fatty Acid Metabolism on Glucose-Induced Changes in Insulin and Glucagon Secretion by Pancreatic Islet Cells.Int J Mol Sci. 2024 May 31;25(11):6052. doi: 10.3390/ijms25116052. Int J Mol Sci. 2024. PMID: 38892240 Free PMC article. Review.
Cited by
-
Glucagon Receptor Signaling and Lipid Metabolism.Front Physiol. 2019 Apr 24;10:413. doi: 10.3389/fphys.2019.00413. eCollection 2019. Front Physiol. 2019. PMID: 31068828 Free PMC article.
-
Epigenome-Wide Association Study of Incident Type 2 Diabetes in a British Population: EPIC-Norfolk Study.Diabetes. 2019 Dec;68(12):2315-2326. doi: 10.2337/db18-0290. Epub 2019 Sep 10. Diabetes. 2019. PMID: 31506343 Free PMC article.
-
Acute Inhibition of Adipose Triglyceride Lipase by NG497 Dysregulates Insulin and Glucagon Secretion From Human Islets.Endocrinology. 2025 May 19;166(7):bqaf090. doi: 10.1210/endocr/bqaf090. Endocrinology. 2025. PMID: 40354133 Free PMC article.
-
Live-cell imaging of glucose-induced metabolic coupling of β and α cell metabolism in health and type 2 diabetes.Commun Biol. 2021 May 19;4(1):594. doi: 10.1038/s42003-021-02113-1. Commun Biol. 2021. PMID: 34012065 Free PMC article.
-
Leucine Suppresses α-Cell cAMP and Glucagon Secretion via a Combination of Cell-Intrinsic and Islet Paracrine Signaling.Diabetes. 2024 Sep 1;73(9):1426-1439. doi: 10.2337/db23-1013. Diabetes. 2024. PMID: 38870025 Free PMC article.
References
-
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Barg S., Galvanovskis J., Göpel S.O., Rorsman P., Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes. 2000;49:1500–1510. - PubMed
-
- Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

