Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster
- PMID: 29898654
- PMCID: PMC6001141
- DOI: 10.1186/s12861-018-0172-6
Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster
Abstract
Background: Pregnant women may be exposed to nicotine if they smoke or use tobacco products, nicotine replacement therapy, or via e-cigarettes. Prenatal nicotine exposure has been shown to have deleterious effects on the nervous system in mammals including changes in brain size and in the dopaminergic system. The genetic and molecular mechanisms for these changes are not well understood. A Drosophila melanogaster model for these effects of nicotine exposure could contribute to faster identification of genes and molecular pathways underlying these effects. The purpose of this study was to determine if developmental nicotine exposure affects the nervous system of Drosophila melanogaster, focusing on changes to brain size and the dopaminergic system at two developmental stages.
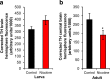
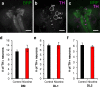
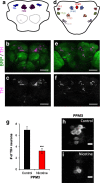
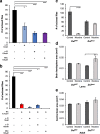
Results: We reared flies on control or nicotine food from egg to 3rd instar larvae or from egg to adult and determined effectiveness of the nicotine treatment. We used immunohistochemistry to visualize the whole brain and dopaminergic neurons, using tyrosine hydroxylase as the marker. We measured brain area, tyrosine hydroxylase fluorescence, and counted the number of dopaminergic neurons in brain clusters. We detected an increase in larval brain hemisphere area, a decrease in tyrosine hydroxylase fluorescence in adult central brains, and a decrease in the number of neurons in the PPM3 adult dopaminergic cluster. We tested involvement of Dα7, one of the nicotinic acetylcholine receptor subunits, and found it was involved in eclosion, as previously described, but not involved in brain size.
Conclusions: We conclude that developmental nicotine exposure in Drosophila melanogaster affects brain size and the dopaminergic system. Prenatal nicotine exposure in mammals has also been shown to have effects on brain size and in the dopaminergic system. This study further establishes Drosophila melanogaster as model organism to study the effects of developmental nicotine exposure. The genetic and molecular tools available for Drosophila research will allow elucidation of the mechanisms underlying the effects of nicotine exposure during development.
Keywords: Brain; Development; Dopamine; Drosophila; Dα7; Nicotine.
Conflict of interest statement
Ethics approval and consent to participate
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress. A report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and PRevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full....
-
- World Health Organization. Tobacco: Fact sheet. 2017. http://www.wpro.who.int/mediacentre/factsheets/fs_201203_tobacco/en/.
-
- Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Substance and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; 2014. https://store.samhsa.gov/product/Results-from-the-2013-National-Survey-o....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

