Comprehensive pharmaceutical care to prevent drug-related readmissions of dependent-living elderly patients: a randomized controlled trial
- PMID: 29898670
- PMCID: PMC6000946
- DOI: 10.1186/s12877-018-0814-3
Comprehensive pharmaceutical care to prevent drug-related readmissions of dependent-living elderly patients: a randomized controlled trial
Abstract
Background: Elderly patients are vulnerable to adverse drug reactions (ADRs). Drug-related readmissions (DRRs) can be a major consequence of ADR. Therefore, this study aimed to investigate the effects of a ward-based, comprehensive pharmaceutical care service on the occurrence of DRRs as the endpoint in dependent-living elderly patients.
Methods: A randomized, controlled trial was performed at a German University Hospital. Patients fulfilling the following criteria were eligible: admission to a cooperating ward, existing drug therapy at admission, 65 years of age and older, home-care or nursing home residents in ambulatory care, and a minimum hospital stay of three days. Patients received either standard care (control group) or pharmaceutical care (intervention group). Follow-up consultations were conducted for each patient at 1, 8, 26, and 52 weeks after discharge. The time to DRR was defined as the primary outcome measure and was analysed using the log-rank test. The Cox-proportional hazard model was used for risk factor analysis.
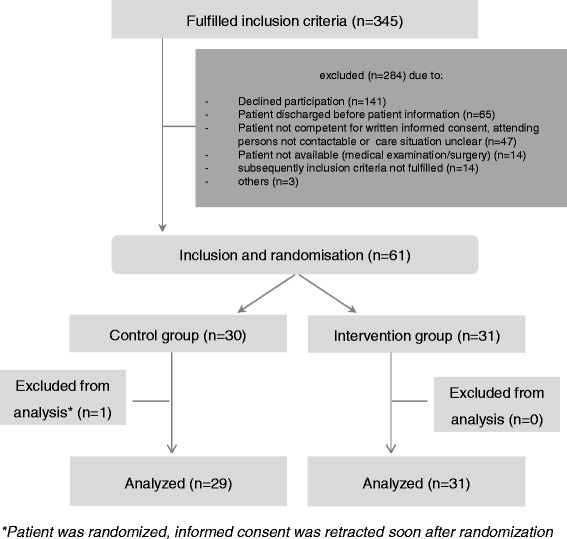
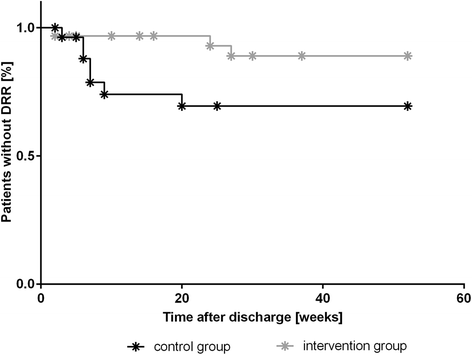
Results: Sixty patients (n = 31 intervention group, n = 29 control group) participated in the study. For patients in the intervention group, the median time to DRR was prolonged; however, the level of statistical significance was not reached (log-rank test P = 0.068; HR = 3.28, P = 0.086). When the risk factors 'age' or 'length of stay on the ward' were added to the Cox proportional hazard model, patients in the control group exhibited a significantly higher risk of experiencing a DRR than patients of the intervention group (HR = 4.62; P = 0.028 including age and HR = 5.76; P = 0.033 including length of stay on the ward).
Conclusions: Our findings demonstrate the successful implementation of ward-based, comprehensive pharmaceutical care for dependent-living elderly. Despite a low participation rate, which led to an underpowered study, the results provide a preliminary efficacy signal and effect size estimates to power a definitive trial.
Trial registration: Clinicaltrials.gov identifier: NCT01578525 , prospectively registered April 13, 2012.
Keywords: Adverse drug reactions; Drug-related readmissions; Elderly patients; Pharmaceutical care.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the local ethics committee (ethic committee RWTH Aachen University, EK 195/12). Written informed consent was obtained from all individual participants or their legal representatives.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous