Disease-specific and general health-related quality of life in newly diagnosed prostate cancer patients: the Pros-IT CNR study
- PMID: 29898750
- PMCID: PMC6001046
- DOI: 10.1186/s12955-018-0952-5
Disease-specific and general health-related quality of life in newly diagnosed prostate cancer patients: the Pros-IT CNR study
Abstract
Background: The National Research Council (CNR) prostate cancer monitoring project in Italy (Pros-IT CNR) is an observational, prospective, ongoing, multicentre study aiming to monitor a sample of Italian males diagnosed as new cases of prostate cancer. The present study aims to present data on the quality of life at time prostate cancer is diagnosed.
Methods: One thousand seven hundred five patients were enrolled. Quality of life is evaluated at the time cancer was diagnosed and at subsequent assessments via the Italian version of the University of California Los Angeles-Prostate Cancer Index (UCLA-PCI) and the Short Form Health Survey (SF-12).
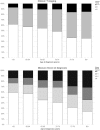
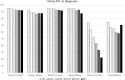
Results: At diagnosis, lower scores on the physical component of the SF-12 were associated to older ages, obesity and the presence of 3+ moderate/severe comorbidities. Lower scores on the mental component were associated to younger ages, the presence of 3+ moderate/severe comorbidities and a T-score higher than one. Urinary and bowel functions according to UCLA-PCI were generally good. Almost 5% of the sample reported using at least one safety pad daily to control urinary loss; less than 3% reported moderate/severe problems attributable to bowel functions, and sexual function was a moderate/severe problem for 26.7%. Diabetes, 3+ moderate/severe comorbidities, T2 or T3-T4 categories and a Gleason score of eight or more were significantly associated with lower sexual function scores at diagnosis.
Conclusions: Data collected by the Pros-IT CNR study have clarified the baseline status of newly diagnosed prostate cancer patients. A comprehensive assessment of quality of life will allow to objectively evaluate outcomes of different profile of care.
Keywords: Diagnosis; Pros-IT CNR study; Prostate cancer; Quality of life.
Conflict of interest statement
Ethics approval and consent to participate
The Pros-IT CNR study protocol was approved by the Ethics Committee of the clinical coordinating center located at the Sant’Anna Hospital (Como, Italy; register number 45/2014). It was also approved by the Ethics Committees of each of the other participating centers.
All participants gave informed consent.
Competing interests
AP, MN, WA, PFB, FB, GNC, RC, MG, SMM, VM, RM, GM, SP, UR, VZ declare that they have no conflicts of interest. AT reports personal fees and other from Astellas, other from Allergan, other from Bayer, other from GSK, personal fees and other from Pierre Fabre, other from Takeda, outside the submitted work. SM and GC report grants from Takeda, during the conduct of the study; personal fees from Takeda, outside the submitted work. Conflict of interest forms for PG, ER and SB were not received.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Global Burden of Disease Collaboration Global, Regional, and national cancer incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability Adjusted Life-years for 32 cancer Groups, 1990 to 2015 A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- AIOM. AIRTUM . I numeri del cancro in Italia. Roma: Il Pensiero Scientifico Editore; 2016.
-
- Velikova G, Coensb C, Efficacec F, Greimeld E, Groenvolde M, Johnsong C, et al. Health-Related Quality of Life in EORTC clinical trials − 30 years of progress from methodological developments to making a real impact on oncology practice. EJC. 2012;Supplements 10(1):141–149.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous