Congenital macrothrombocytopenia with focal myelofibrosis due to mutations in human G6b-B is rescued in humanized mice
- PMID: 29898956
- PMCID: PMC6161765
- DOI: 10.1182/blood-2017-08-802769
Congenital macrothrombocytopenia with focal myelofibrosis due to mutations in human G6b-B is rescued in humanized mice
Abstract
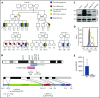
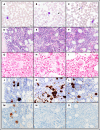
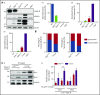
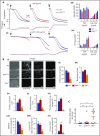
Unlike primary myelofibrosis (PMF) in adults, myelofibrosis in children is rare. Congenital (inherited) forms of myelofibrosis (cMF) have been described, but the underlying genetic mechanisms remain elusive. Here we describe 4 families with autosomal recessive inherited macrothrombocytopenia with focal myelofibrosis due to germ line loss-of-function mutations in the megakaryocyte-specific immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptor G6b-B (G6b, C6orf25, or MPIG6B). Patients presented with a mild-to-moderate bleeding diathesis, macrothrombocytopenia, anemia, leukocytosis and atypical megakaryocytes associated with a distinctive, focal, perimegakaryocytic pattern of bone marrow fibrosis. In addition to identifying the responsible gene, the description of G6b-B as the mutated protein potentially implicates aberrant G6b-B megakaryocytic signaling and activation in the pathogenesis of myelofibrosis. Targeted insertion of human G6b in mice rescued the knockout phenotype and a copy number effect of human G6b-B expression was observed. Homozygous knockin mice expressed 25% of human G6b-B and exhibited a marginal reduction in platelet count and mild alterations in platelet function; these phenotypes were more severe in heterozygous mice that expressed only 12% of human G6b-B. This study establishes G6b-B as a critical regulator of platelet homeostasis in humans and mice. In addition, the humanized G6b mouse will provide an invaluable tool for further investigating the physiological functions of human G6b-B as well as testing the efficacy of drugs targeting this receptor.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
G6b-B: the "Y's" and wherefores.Blood. 2018 Sep 27;132(13):1359-1360. doi: 10.1182/blood-2018-06-858738. Blood. 2018. PMID: 30262581 Free PMC article.
References
-
- Carroll WL, Berberich FR, Glader BE. Pancytopenia with myelofibrosis. An unusual presentation of childhood Hodgkin’s disease. Clin Pediatr (Phila). 1986;25(2):106-108. - PubMed
-
- Balkan C, Ersoy B, Nese N. Myelofibrosis associated with severe vitamin D deficiency rickets. J Int Med Res. 2005;33(3):356-359. - PubMed
-
- al-Eissa YA, al-Mashhadani SA. Myelofibrosis in severe combined immunodeficiency due to vitamin D deficiency rickets. Acta Haematol. 1994;92(3):160-163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

