Probiotic strains detect and suppress cholera in mice
- PMID: 29899022
- PMCID: PMC7821980
- DOI: 10.1126/scitranslmed.aao2586
Probiotic strains detect and suppress cholera in mice
Abstract
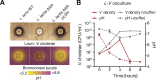
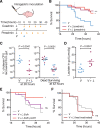
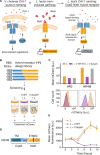
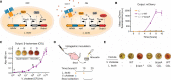
Microbiota-modulating interventions are an emerging strategy to promote gastrointestinal homeostasis. Yet, their use in the detection, prevention, and treatment of acute infections remains underexplored. We report the basis of a probiotic-based strategy to promote colonization resistance and point-of-need diagnosis of cholera, an acute diarrheal disease caused by the pathogen Vibrio cholerae Oral administration of Lactococcus lactis, a common dietary fermentative bacterium, reduced intestinal V. cholerae burden and improved survival in infected infant mice through the production of lactic acid. Furthermore, we engineered an L. lactis strain that specifically detects quorum-sensing signals of V. cholerae in the gut and triggers expression of an enzymatic reporter that is readily detected in fecal samples. We postulate that preventive dietary interventions with fermented foods containing natural and engineered L. lactis strains may hinder cholera progression and improve disease surveillance in populations at risk of cholera outbreaks.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

Comment in
-
Curbing cholera.Sci Transl Med. 2018 Jun 13;10(445):eaat9483. doi: 10.1126/scitranslmed.aat9483. Sci Transl Med. 2018. PMID: 29899025
References
-
- Marteau P. R, de Vrese M, Cellier C. J, Schrezenmeir J, Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 73, 430S–436S (2001). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

