Role of Microtubules and Microtubule-Associated Proteins in HIV-1 Infection
- PMID: 29899089
- PMCID: PMC6069196
- DOI: 10.1128/JVI.00085-18
Role of Microtubules and Microtubule-Associated Proteins in HIV-1 Infection
Abstract
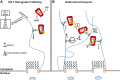
Recent studies show that human immunodeficiency virus type 1 (HIV-1) can utilize microtubules and their associated proteins to complete key postfusion steps during infection. These include associating with both dynein and kinesin motors, as well as proteins, which enhance infection by altering microtubule dynamics during infection. In this article, we will discuss findings on how dynein and kinesin motors, as well as other microtubule-associated proteins, influence HIV-1 trafficking, viral core uncoating, and nuclear import of the viral ribonucleoprotein (RNP).
Keywords: BICD2; EB1; FEZ1; HIV-1; dynein; kinesin-1; microtubules.
Copyright © 2018 American Society for Microbiology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

