Broadly Reactive Human Monoclonal Antibodies Elicited following Pandemic H1N1 Influenza Virus Exposure Protect Mice against Highly Pathogenic H5N1 Challenge
- PMID: 29899095
- PMCID: PMC6069173
- DOI: 10.1128/JVI.00949-18
Broadly Reactive Human Monoclonal Antibodies Elicited following Pandemic H1N1 Influenza Virus Exposure Protect Mice against Highly Pathogenic H5N1 Challenge
Abstract
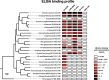
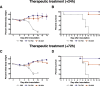
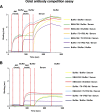
Broadly cross-reactive antibodies (Abs) that recognize conserved epitopes within the influenza virus hemagglutinin (HA) stalk domain are of particular interest for their potential use as therapeutic and prophylactic agents against multiple influenza virus subtypes, including zoonotic virus strains. Here, we characterized four human HA stalk-reactive monoclonal antibodies (MAbs) for their binding breadth and affinity, in vitro neutralization capacity, and in vivo protective potential against an highly pathogenic avian influenza virus. The monoclonal antibodies were isolated from individuals shortly following infection with (70-1F02 and 1009-3B05) or vaccination against (05-2G02 and 09-3A01) A(H1N1)pdm09. Three of the MAbs bound HAs from multiple strains of group 1 viruses, and one MAb, 05-2G02, bound to both group 1 and group 2 influenza A virus HAs. All four antibodies prophylactically protected mice against a lethal challenge with the highly pathogenic A/Vietnam/1203/04 (H5N1) strain. Two MAbs, 70-1F02 and 09-3A01, were further tested for their therapeutic efficacy against the same strain and showed good efficacy in this setting as well. One MAb, 70-1F02, cocrystallized with H5 HA and showed heavy-chain-only interactions similar to those seen with the previously described CR6261 anti-stalk antibody. Finally, we show that antibodies that compete with these MAbs are prevalent in serum from an individual recently infected with the A(H1N1)pdm09 virus. The antibodies described here can be developed into broad-spectrum antiviral therapeutics that could be used to combat infections by zoonotic or emerging pandemic influenza viruses.IMPORTANCE The rise in zoonotic infections of humans by emerging influenza viruses is a worldwide public health concern. The majority of recent zoonotic human influenza cases were caused by H7N9 and H5Nx viruses and were associated with high morbidity and mortality. In addition, seasonal influenza viruses are estimated to cause up to 650,000 deaths annually worldwide. Currently available antiviral treatment options include only neuraminidase inhibitors, but some influenza viruses are naturally resistant to these drugs, and others quickly develop resistance-conferring mutations. Alternative therapeutics are urgently needed. Broadly protective antibodies that target the conserved "stalk" domain of the hemagglutinin represent potential potent antiviral prophylactic and therapeutic agents that can assist pandemic preparedness. Here, we describe four human monoclonal antibodies that target conserved regions of influenza HA and characterize their binding spectrum as well as their protective capacity in prophylactic and therapeutic settings against a lethal challenge with a zoonotic influenza virus.
Keywords: H5N1; HA stalk; hemagglutinin; hemagglutinin stalk; influenza; influenza virus; monoclonal antibody.
Copyright © 2018 American Society for Microbiology.
Figures




Similar articles
-
Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells.PLoS One. 2008;3(12):e3942. doi: 10.1371/journal.pone.0003942. Epub 2008 Dec 16. PLoS One. 2008. PMID: 19079604 Free PMC article.
-
Hemagglutinin Stalk- and Neuraminidase-Specific Monoclonal Antibodies Protect against Lethal H10N8 Influenza Virus Infection in Mice.J Virol. 2015 Oct 28;90(2):851-61. doi: 10.1128/JVI.02275-15. Print 2016 Jan 15. J Virol. 2015. PMID: 26512088 Free PMC article.
-
Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses.J Virol. 2013 Aug;87(16):9290-300. doi: 10.1128/JVI.01203-13. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785204 Free PMC article.
-
Anti-neuraminidase immunity in the combat against influenza.Expert Rev Vaccines. 2024 Jan-Dec;23(1):474-484. doi: 10.1080/14760584.2024.2343689. Epub 2024 Apr 23. Expert Rev Vaccines. 2024. PMID: 38632930 Free PMC article. Review.
-
Cross-reactive humoral responses to influenza and their implications for a universal vaccine.Ann N Y Acad Sci. 2013 Apr;1283:13-21. doi: 10.1111/nyas.12012. Epub 2013 Feb 13. Ann N Y Acad Sci. 2013. PMID: 23405860 Review.
Cited by
-
FLU-LISA (fluorescence-linked immunosorbent assay): high-throughput antibody profiling using antigen microarrays.Immunol Cell Biol. 2023 Mar;101(3):231-248. doi: 10.1111/imcb.12618. Epub 2023 Jan 29. Immunol Cell Biol. 2023. PMID: 36567516 Free PMC article.
-
SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2.Cell. 2021 Jul 22;184(15):3936-3948.e10. doi: 10.1016/j.cell.2021.06.005. Epub 2021 Jun 8. Cell. 2021. PMID: 34192529 Free PMC article.
-
Harnessing immunotherapeutic molecules and diagnostic biomarkers as human-derived adjuvants for MERS-CoV vaccine development.Front Immunol. 2025 Mar 13;16:1538301. doi: 10.3389/fimmu.2025.1538301. eCollection 2025. Front Immunol. 2025. PMID: 40181980 Free PMC article. Review.
-
SARS-CoV-2 Omicron boosting induces de novo B cell response in humans.bioRxiv [Preprint]. 2022 Sep 22:2022.09.22.509040. doi: 10.1101/2022.09.22.509040. bioRxiv. 2022. Update in: Nature. 2023 May;617(7961):592-598. doi: 10.1038/s41586-023-06025-4. PMID: 36172127 Free PMC article. Updated. Preprint.
-
A public vaccine-induced human antibody protects against SARS-CoV-2 and emerging variants.bioRxiv [Preprint]. 2021 Mar 24:2021.03.24.436864. doi: 10.1101/2021.03.24.436864. bioRxiv. 2021. Update in: Immunity. 2021 Sep 14;54(9):2159-2166.e6. doi: 10.1016/j.immuni.2021.08.013. PMID: 33791696 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. 2009. WHO fact sheet no. 211: Influenza. http://www.who.int/mediacentre/factsheets/fs211/en/index.html. World Health Organization, Geneva, Switzerland.
-
- Jiang H, Wu P, Uyeki TM, He J, Deng Z, Xu W, Lv Q, Zhang J, Wu Y, Tsang TK, Kang M, Zheng J, Wang L, Yang B, Qin Y, Feng L, Fang VJ, Gao GF, Leung GM, Yu H, Cowling BJ. 1 August 2017. Preliminary epidemiologic assessment of human infections with highly pathogenic avian influenza A(H5N6) virus, China. Clin Infect Dis doi:10.1093/cid/cix334. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

