Probing the correlation between insulin activity and structural stability through introduction of the rigid A6-A11 bond
- PMID: 29899115
- PMCID: PMC6066309
- DOI: 10.1074/jbc.RA118.002486
Probing the correlation between insulin activity and structural stability through introduction of the rigid A6-A11 bond
Abstract
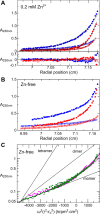
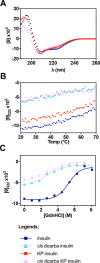
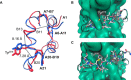
The development of fast-acting and highly stable insulin analogues is challenging. Insulin undergoes structural transitions essential for binding and activation of the insulin receptor (IR), but these conformational changes can also affect insulin stability. Previously, we substituted the insulin A6-A11 cystine with a rigid, non-reducible C=C linkage ("dicarba" linkage). A cis-alkene permitted the conformational flexibility of the A-chain N-terminal helix necessary for high-affinity IR binding, resulting in surprisingly rapid activity in vivo Here, we show that, unlike the rapidly acting LysB28ProB29 insulin analogue (KP insulin), cis-dicarba insulin is not inherently monomeric. We also show that cis-dicarba KP insulin lowers blood glucose levels even more rapidly than KP insulin, suggesting that an inability to oligomerize is not responsible for the observed rapid activity onset of cis-dicarba analogues. Although rapid-acting, neither dicarba species is stable, as assessed by fibrillation and thermodynamics assays. MALDI analyses and molecular dynamics simulations of cis-dicarba insulin revealed a previously unidentified role of the A6-A11 linkage in insulin conformational dynamics. By controlling the conformational flexibility of the insulin B-chain helix, this linkage affects overall insulin structural stability. This effect is independent of its regulation of the A-chain N-terminal helix flexibility necessary for IR engagement. We conclude that high-affinity IR binding, rapid in vivo activity, and insulin stability can be regulated by the specific conformational arrangement of the A6-A11 linkage. This detailed understanding of insulin's structural dynamics may aid in the future design of rapid-acting insulin analogues with improved stability.
Keywords: biophysical studies; biophysics; conformational change; dicarba peptides; disulfide; disulfide bonds; insulin; molecular dynamics.
© 2018 Ong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Banting F. G., and Best C. H. (2007) The internal secretion of the pancreas. 1922. Indian J. Med. Res. 125, 251–266 - PubMed
-
- Adams M. J., Blundell T. L., Dodson E. J., Dodson G. G., Vijayan M., Baker E. N., Harding M. M., Hodgkin D. C., Rimmer B., and Sheat S. (1969) Structure of rhombohedral 2 zinc insulin crystals. Nature 224, 491–495 10.1038/224491a0 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

