Molecular Pathways Underlying Cholesterol Homeostasis
- PMID: 29899250
- PMCID: PMC6024674
- DOI: 10.3390/nu10060760
Molecular Pathways Underlying Cholesterol Homeostasis
Abstract
Cholesterol is an essential molecule that exerts pleiotropic actions. Although its presence is vital to the cell, its excess can be harmful and, therefore, sustaining cholesterol homeostasis is crucial to maintaining proper cellular functioning. It is well documented that high plasma cholesterol concentration increases the risk of atherosclerotic heart disease. In the last decades, several studies have investigated the association of plasma cholesterol concentrations and the risk of cardiovascular diseases as well as the signaling pathways involved in cholesterol homeostasis. Here, we present an overview of several mechanisms involved in intestinal cholesterol absorption, the regulation of cholesterol synthesis and uptake. We also discuss the importance of reverse cholesterol transport and transintestinal cholesterol transport to maintain cholesterol homeostasis and prevent atherosclerosis development. Additionally, we discuss the influence of dietary cholesterol on plasma cholesterol concentration and the new recommendations for cholesterol intake in a context of a healthy dietary pattern.
Keywords: cardiovascular disease; cholesterol; cholesterol homeostasis; dietary cholesterol; molecular pathways.
Conflict of interest statement
The authors declare no competing interest
Figures
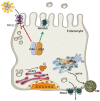


References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

