Neuronal Nicotinic Acetylcholine Receptor Modulators from Cone Snails
- PMID: 29899286
- PMCID: PMC6024932
- DOI: 10.3390/md16060208
Neuronal Nicotinic Acetylcholine Receptor Modulators from Cone Snails
Abstract
Marine cone snails are a large family of gastropods that have evolved highly potent venoms for predation and defense. The cone snail venom has exceptional molecular diversity in neuropharmacologically active compounds, targeting a range of receptors, ion channels, and transporters. These conotoxins have helped to dissect the structure and function of many of these therapeutically significant targets in the central and peripheral nervous systems, as well as unravelling the complex cellular mechanisms modulated by these receptors and ion channels. This review provides an overview of α-conotoxins targeting neuronal nicotinic acetylcholine receptors. The structure and activity of both classical and non-classical α-conotoxins are discussed, along with their contributions towards understanding nicotinic acetylcholine receptor (nAChR) structure and function.
Keywords: conotoxins; nicotinic acetylcholine receptors; α-conotoxins.
Conflict of interest statement
The authors declare no conflict of interest
Figures
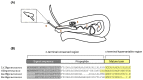
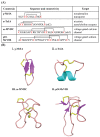
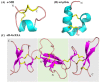

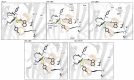
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

