Addition of intraperitoneal cisplatin and etoposide to first-line chemotherapy for advanced ovarian cancer: a randomised, phase 2 trial
- PMID: 29899395
- PMCID: PMC6035193
- DOI: 10.1038/s41416-018-0036-7
Addition of intraperitoneal cisplatin and etoposide to first-line chemotherapy for advanced ovarian cancer: a randomised, phase 2 trial
Abstract
Background: We assessed the efficacy of adding intraperitoneal (IP) chemotherapy to standard first-line intravenous (IV) chemotherapy in epithelial ovarian cancer (EOC) patients.
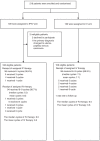
Methods: Patients with stage IIIC-IV EOC who underwent optimal debulking surgery were randomly assigned to four cycles of weekly IP chemotherapy with cisplatin (50 mg/m2) and etoposide (100 mg/m2) followed by six cycles of IV chemotherapy every 3 weeks (IP/IV arm), or were administered IV chemotherapy alone (IV arm). The primary endpoint for this study was the 12-month non-progression rate (NPR).
Results: Between 4/2009 and 9/2015, 218 patients were randomised, of whom 215 initiated treatment. In the IP/IV arm, 90.6% of patients completed 4 cycles of IP chemotherapy. The 12-month NPRs were 81.9% and 64.2% in the IP/IV and IV groups, respectively (HR 0.48 (95% CI 0.27-0.82)). The median progression-free survival (PFS) was increased in the IP/IV arm compared with that in the IV arm (22.4 vs. 16.8 months; HR 0.66 (0.48-0.91)) and in a subgroup with no gross cytoreduction (31.1 vs. 16.8 months; HR 0.46 (0.26-0.82)). Similar findings were detected with regard to time to first subsequent anticancer therapy (TFST) (25.9 vs. 18.0 months; P = 0.009) and time to second subsequent anticancer therapy (TSST) (40.8 vs. 30.1 months; P = 0.042). Grade 3/4 leukopenia, anaemia and gastrointestinal events were more common in the IP/IV arm, but the treatment burden was considered acceptable.
Conclusions: IP chemotherapy prior to IV chemotherapy was associated with an increased 12-month NPR and a longer TSST than IV alone in patients with EOC, albeit with acceptable toxic effects. Long-term follow-up is warranted to identify the effects of IP therapy on overall survival.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Survival benefits of dose-dense early postoperative intraperitoneal chemotherapy in front-line therapy for advanced ovarian cancer: a randomised controlled study.Br J Cancer. 2019 Aug;121(5):425-428. doi: 10.1038/s41416-019-0543-1. Epub 2019 Aug 6. Br J Cancer. 2019. PMID: 31383985 Free PMC article. Clinical Trial.
-
Timing is everything: intraperitoneal chemotherapy after primary or interval debulking surgery for advanced ovarian cancer.Cancer Chemother Pharmacol. 2018 Jul;82(1):55-63. doi: 10.1007/s00280-018-3591-y. Epub 2018 Apr 27. Cancer Chemother Pharmacol. 2018. PMID: 29704010
-
Evaluation of non-completion of intraperitoneal chemotherapy in patients with advanced epithelial ovarian cancer.J Gynecol Oncol. 2019 Nov;30(6):e93. doi: 10.3802/jgo.2019.30.e93. J Gynecol Oncol. 2019. PMID: 31576687 Free PMC article.
-
Intraperitoneal chemotherapy for ovarian cancer: where are we now?J Natl Compr Canc Netw. 2006 Oct;4(9):941-6. doi: 10.6004/jnccn.2006.0077. J Natl Compr Canc Netw. 2006. PMID: 17020670 Review.
-
The Evolving Landscape of Chemotherapy in Newly Diagnosed Advanced Epithelial Ovarian Cancer.Am Soc Clin Oncol Educ Book. 2019 Jan;39:e141-e151. doi: 10.1200/EDBK_239007. Epub 2019 May 17. Am Soc Clin Oncol Educ Book. 2019. PMID: 31099631 Review.
Cited by
-
Asian Society of Gynecologic Oncology International Workshop 2018.J Gynecol Oncol. 2019 Mar;30(2):e39. doi: 10.3802/jgo.2019.30.e39. Epub 2019 Jan 14. J Gynecol Oncol. 2019. PMID: 30740961 Free PMC article.
-
The acute toxic effects of platinum nanoparticles on ion channels, transmembrane potentials of cardiomyocytes in vitro and heart rhythm in vivo in mice.Int J Nanomedicine. 2019 Jul 22;14:5595-5609. doi: 10.2147/IJN.S209135. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31413565 Free PMC article.
-
Feasibility of neoadjuvant and adjuvant intraperitoneal chemotherapy in patients with advanced epithelial ovarian cancer: A single-center experience.Medicine (Baltimore). 2020 Sep 4;99(36):e22100. doi: 10.1097/MD.0000000000022100. Medicine (Baltimore). 2020. PMID: 32899091 Free PMC article.
-
Survival benefits of dose-dense early postoperative intraperitoneal chemotherapy in front-line therapy for advanced ovarian cancer: a randomised controlled study.Br J Cancer. 2019 Aug;121(5):425-428. doi: 10.1038/s41416-019-0543-1. Epub 2019 Aug 6. Br J Cancer. 2019. PMID: 31383985 Free PMC article. Clinical Trial.
-
Apoptotic mechanism activated by blue light and cisplatinum in cutaneous squamous cell carcinoma cells.Int J Mol Med. 2021 Apr;47(4):48. doi: 10.3892/ijmm.2021.4881. Epub 2021 Feb 12. Int J Mol Med. 2021. PMID: 33576463 Free PMC article.
References
-
- Markman M, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. - DOI - PubMed
-
- National Cancer Institute Announcement (2006): Intraperitoneal chemotherapy for ovarian cancer. Cancer Therapy Evaluation Program [online]: http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

