Structure of the µ-opioid receptor-Gi protein complex
- PMID: 29899455
- PMCID: PMC6317904
- DOI: 10.1038/s41586-018-0219-7
Structure of the µ-opioid receptor-Gi protein complex
Abstract
The μ-opioid receptor (μOR) is a G-protein-coupled receptor (GPCR) and the target of most clinically and recreationally used opioids. The induced positive effects of analgesia and euphoria are mediated by μOR signalling through the adenylyl cyclase-inhibiting heterotrimeric G protein Gi. Here we present the 3.5 Å resolution cryo-electron microscopy structure of the μOR bound to the agonist peptide DAMGO and nucleotide-free Gi. DAMGO occupies the morphinan ligand pocket, with its N terminus interacting with conserved receptor residues and its C terminus engaging regions important for opioid-ligand selectivity. Comparison of the μOR-Gi complex to previously determined structures of other GPCRs bound to the stimulatory G protein Gs reveals differences in the position of transmembrane receptor helix 6 and in the interactions between the G protein α-subunit and the receptor core. Together, these results shed light on the structural features that contribute to the Gi protein-coupling specificity of the µOR.
Conflict of interest statement
The authors declare one competing interest: Brian Kobilka is a founder of and consultant for ConfometRx, Inc. Readers are welcome to comment on the online version of the paper.
Figures
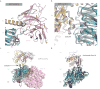

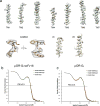
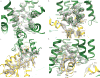
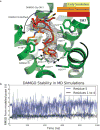


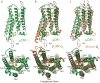
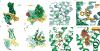
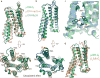
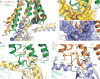
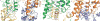
Comment in
-
How the ubiquitous GPCR receptor family selectively activates signalling pathways.Nature. 2018 Jun;558(7711):529-530. doi: 10.1038/d41586-018-05503-4. Nature. 2018. PMID: 29946098 No abstract available.
References
-
- Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. - PubMed
-
- Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. - PubMed
-
- Raffa RB, Martinez RP, Connelly CD. G-protein antisense oligodeoxyribonucleotides and mu-opioid supraspinal antinociception. Eur J Pharmacol. 1994;258:R5–7. - PubMed
-
- Raehal KM, Walker JKL, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

