Imaging EGFR and HER3 through 89Zr-labeled MEHD7945A (Duligotuzumab)
- PMID: 29899472
- PMCID: PMC5998059
- DOI: 10.1038/s41598-018-27454-6
Imaging EGFR and HER3 through 89Zr-labeled MEHD7945A (Duligotuzumab)
Abstract
Tumor resistance to treatment paved the way toward the development of single agent drugs that target multiple molecular signatures amplified within the malignancy. The discovered crosstalk between EGFR and HER3 as well as the role of HER3 in mediating EGFR resistance made these two receptor tyrosine kinases attractive targets. MEHD7945A or duligotuzumab is a single immunotherapy agent that dually targets both molecular signatures. In this study, a positron emission tomography (PET) companion diagnostic to MEHD7945A is reported and evaluated in pancreatic cancer. Tumor accretion and whole body pharmacokinetics of 89Zr-MEHD7945A were established. Specificity of the probe for EGFR and/or HER3 was further examined.
Conflict of interest statement
The authors declare no competing interests.
Figures

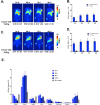
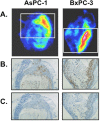
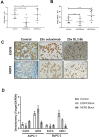
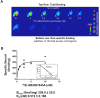
References
-
- Dancer, J., Takei, H., Ro, J. Y. & Lowery-Nordberg, M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: A comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol. Rep. (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

