Soft transparent graphene contact lens electrodes for conformal full-cornea recording of electroretinogram
- PMID: 29899545
- PMCID: PMC5998030
- DOI: 10.1038/s41467-018-04781-w
Soft transparent graphene contact lens electrodes for conformal full-cornea recording of electroretinogram
Abstract
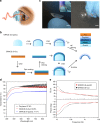
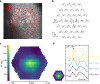
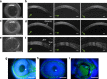
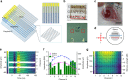
Visual electrophysiology measurements are important for ophthalmic diagnostic testing. Electrodes with combined optical transparency and softness are highly desirable, and sometimes indispensable for many ocular electrophysiology measurements. Here we report the fabrication of soft graphene contact lens electrodes (GRACEs) with broad-spectrum optical transparency, and their application in conformal, full-cornea recording of electroretinography (ERG) from cynomolgus monkeys. The GRACEs give higher signal amplitude than conventional ERG electrodes in recordings of various full-field ERG responses. High-quality topographic mapping of multifocal ERG under simultaneous fundus monitoring is realized. A conformal and tight interface between the GRACEs and cornea is revealed. Neither corneal irritation nor abnormal behavior of the animals is observed after ERG measurements with GRACEs. Furthermore, spatially resolved ERG recordings on rabbits with graphene multi-electrode array reveal a stronger signal at the central cornea than the periphery. These results demonstrate the unique capabilities of the graphene-based electrodes for in vivo visual electrophysiology studies.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Heckenlively, J. R. & Arden, G. B. Principles and practice of clinical electrophysiology of vision. (MIT press, Cambridge, MA, 2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

