Tetracycline-Inactivating Enzymes
- PMID: 29899733
- PMCID: PMC5988894
- DOI: 10.3389/fmicb.2018.01058
Tetracycline-Inactivating Enzymes
Abstract
Tetracyclines have been foundational antibacterial agents for more than 70 years. Renewed interest in tetracycline antibiotics is being driven by advancements in tetracycline synthesis and strategic scaffold modifications designed to overcome established clinical resistance mechanisms including efflux and ribosome protection. Emerging new resistance mechanisms, including enzymatic antibiotic inactivation, threaten recent progress on bringing these next-generation tetracyclines to the clinic. Here we review the current state of knowledge on the structure, mechanism, and inhibition of tetracycline-inactivating enzymes.
Keywords: antibiotic adjuvants; antibiotic resistance; enzymatic antibiotic inactivation; flavin monooxygenase; tetracycline destructases; tetracyclines.
Figures




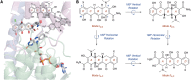
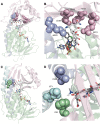



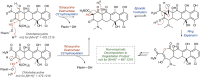
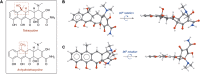
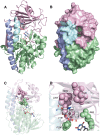

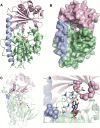
References
-
- Bolam D. N., Roberts S., Proctor M. R., Turkenburg J. P., Dodson E. J., Martinez-Fleites C., et al. (2007). The crystal structure of two macrolide glycosyltransferases provides a blueprint for host cell antibiotic immunity. Proc. Natl. Acad. Sci. U.S.A. 104 5336–5341. 10.1073/pnas.0607897104 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

