Alpha-1 Antitrypsin Attenuates M1 Microglia-Mediated Neuroinflammation in Retinal Degeneration
- PMID: 29899745
- PMCID: PMC5988858
- DOI: 10.3389/fimmu.2018.01202
Alpha-1 Antitrypsin Attenuates M1 Microglia-Mediated Neuroinflammation in Retinal Degeneration
Abstract
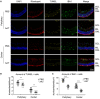
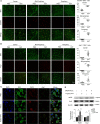
Neurodegenerative diseases are a set of disorders characterized by progressive neuronal death and are associated with microglia-mediated neuroinflammation. Recently, neuroinflammation is proposed as a promising therapeutic target for many neurodegenerative diseases. Alpha-1 antitrypsin (AAT) is recognized as a novel immunomodulatory agent in autoimmune diseases and transplantation, however, its impact on neuroinflammation and neurodegeneration remains unknown. This study aims to explore the effects of AAT on microglia-mediated neuroinflammation and retinal degeneration in rd1 mouse model. We found reduced expression of AAT in rd1 retina, and AAT supplement exhibited certain protective effect on retinal degeneration, presenting with increased amount of photoreceptor nuclei, and amplified wave amplitudes in electroretinogram analysis. Of note, AAT shifted microglia phenotype from pro-inflammatory M1 (CD16/CD32+, iNOS+) to anti-inflammatory M2 (CD206+, Arg1+) both in vivo and in vitro, underscoring the concept of immunomodulation on microglia polarization by AAT during neurodegeneration. Furthermore, AAT suppressed the activation of STAT1, promoted the expression of IRF4 while inhibited IRF8 expression, indicating the involvement of these signaling pathways in AAT immunomodulation. Collectively, our data provided evidence for a novel protective role of AAT through immunomodulation on microglia polarization. Attenuating neuroinflammation by AAT may be beneficial to retard neurodegeneration in rd1 mice.
Keywords: alpha-1 antitrypsin; immunomodulation; microglia polarization; neuroinflammation; rd1 mice.
Figures
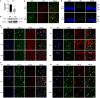
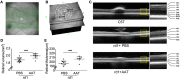
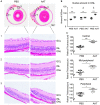
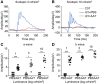


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

