Role of p27Kip1 as a transcriptional regulator
- PMID: 29899857
- PMCID: PMC5995243
- DOI: 10.18632/oncotarget.25447
Role of p27Kip1 as a transcriptional regulator
Abstract
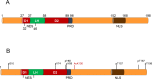
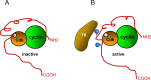
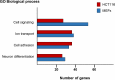
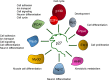
The protein p27Kip1 is a member of the Cip/Kip family of cyclin-dependent kinase (Cdk) inhibitors. It interacts with both the catalytic and the regulatory subunit (cyclin) and introduces a region into the catalytic cleave of the Cdk inducing its inactivation. Its inhibitory capacity can be modulated by specific tyrosine phosphorylations. p27Kip1 also behaves as a transcriptional regulator. It associates with specific chromatin domains through different transcription factors. ChIP on chip, ChIP-seq and expression microarray analysis allowed the identification of the transcriptional programs regulated by p27Kip1. Thus, important cellular functions as cell division cycle, respiration, RNA processing, translation and cell adhesion, are under p27Kip1 regulation. Moreover, genes involved in pathologies as cancer and neurodegeneration are also regulated by p27Kip1, suggesting its implication in these pathologies. The carboxyl moiety of p27Kip1 can associate with different proteins, including transcriptional regulators. In contrast, its NH2-terminal region specifically interacts with cyclin-Cdk complexes. The general mechanistic model of how p27Kip1 regulates transcription is that it associates by its COOH region to the transcriptional regulators on the chromatin and by the NH2-domain to cyclin-Cdk complexes. After Cdk activation it would phosphorylate the specific targets on the chromatin leading to gene expression. This model has been demonstrated to apply in the transcriptional regulation of p130/E2F4 repressed genes involved in cell cycle progression. We summarize in this review our current knowledge on the role of p27Kip1 in the regulation of transcription, on the transcriptional programs under its regulation and on its relevance in pathologies as cancer and neurodegeneration.
Keywords: cancer; neurodegeneration; p27Kip1; transcriptional regulation.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–69. - PubMed
-
- Chang B, Zheng SL, Isaacs SD, Wiley KE, Turner A, Li G, Walsh PC, Meyers DA, Isaacs WB, Xu J. A polymorphism in the CDKN1B gene is associated with increased risk of hereditary prostate cancer. Cancer Res. 2004;64:1997–9. - PubMed
-
- Bencivenga D, Caldarelli I, Stampone E, Mancini FP, Balestrieri ML, Della Ragione F, Borriello A. p27 Kip1 and human cancers: A reappraisal of a still enigmatic protein. Cancer Lett. 2017;403:354–65. - PubMed
-
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. - PubMed
-
- Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269:2–12. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

