Electrochemical imaging of cells and tissues
- PMID: 29899947
- PMCID: PMC5969511
- DOI: 10.1039/c8sc01035h
Electrochemical imaging of cells and tissues
Abstract
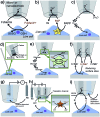
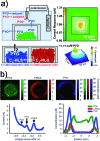
The technological and experimental progress in electrochemical imaging of biological specimens is discussed with a view on potential applications for skin cancer diagnostics, reproductive medicine and microbial testing. The electrochemical analysis of single cell activity inside cell cultures, 3D cellular aggregates and microtissues is based on the selective detection of electroactive species involved in biological functions. Electrochemical imaging strategies, based on nano/micrometric probes scanning over the sample and sensor array chips, respectively, can be made sensitive and selective without being affected by optical interference as many other microscopy techniques. The recent developments in microfabrication, electronics and cell culturing/tissue engineering have evolved in affordable and fast-sampling electrochemical imaging platforms. We believe that the topics discussed herein demonstrate the applicability of electrochemical imaging devices in many areas related to cellular functions.
Figures




References
-
- Polcari D., Dauphin-Ducharme P., Mauzeroll J. Chem. Rev. 2016;116:13234–13278. - PubMed
-
- Hansma P. K., Drake B., Marti O., Gould S. A. C., Prater C. B. Science. 1989;243:641–643. - PubMed
-
- Clausmeyer J., Schuhmann W. Trends Anal. Chem. 2016;79:46–59.
-
- Kwak J., Bard A. J. Anal. Chem. 1989;61:1221–1227.
-
- Zhao C., Wittstock G. Angew. Chem., Int. Ed. 2004;43:4170–4172. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

