Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy
- PMID: 29900038
- PMCID: PMC5993493
- DOI: 10.1080/2162402X.2018.1438111
Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy
Abstract
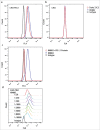
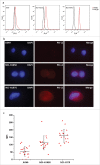
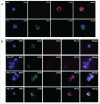
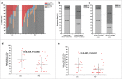
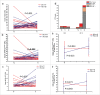
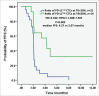
Background: Tumor PD-L1 levels have predictive value in PD-1/PD-L1 checkpoint blockade therapies, yet biopsies can only provide baseline information. Whether PD-L1 expression on circulating tumor cells (CTCs) could serve as an alternative biomarker is of great interest. Design: We established an immunofluorescence assay for semi-quantitative assessment of the PD-L1 expression levels on CTCs with four categories (PD-L1negative, PD-L1low, PD-L1medium and PD-L1high). 35 patients with different advanced gastrointestinal tumors were enrolled in a phase 1 trial of a PD-1 inhibitor, IBI308. The CTC numeration and the PD-L1 expression levels were analyzed. Results: Prior the treatment of IBI308, 97% (34/35) patients had CTCs, ranging from1 to 70 (median 7). 74% (26/35) had PD-L1positive CTCs, and 60% (21/35) had at least one PD-L1high CTCs. The disease control (DC) rate in PD-L1high patients (48%) is much higher than the others (14%). The group with at least 20% abundance of PD-L1high CTCs had even higher DC rate of 64% (9/14), with only 14% DC rate for the rest (3/21). We also observed that the count changes of total CTC, PD-L1postive CTC and PD-L1high CTC correlate quite well with disease outcome (P<0.001, P = 0.002 and 0.007, respectively). In addition, the abundance of PD-L1high CTCs at baseline had predicative significance for progression free survival (PFS). Conclusions: We revealed that the abundance of PD-L1high CTCs at baseline might serve as a predictor to screen patients for PD-1/PD-L1 blockade therapies and measuring the dynamic changes of CTC could indicate the therapeutic response at early time.
Keywords: advanced solid tumor; circulating tumor cells (CTCs); immunotherapy; programmed death-ligand 1 (PD-L1); semi-quantitative analysis.
Figures

References
-
- Weinstock C, Khozin S, Suzman D, Zhang L, Tang S, Wahby S, Goldberg KB, Kim G, Pazdur R. U.S. food and drug administration approval summary: Atezolizumab for metastatic non-small cell lung cancer. Clin Cancer Res. 2017;23(16):4534–4539. doi:10.1158/1078-0432.CCR-17-0540. PMID:28611199. - DOI - PubMed
-
- Maraz A, Geczi L. [The role of immunotherapy in the modern treatment of urothelial carcinoma]. Magyar Onkologia. 2017;61:139–46. PMID:28585615. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
