Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma
- PMID: 29900046
- PMCID: PMC5993498
- DOI: 10.1080/2162402X.2018.1440930
Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma
Abstract
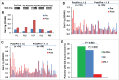
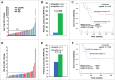
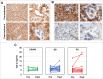
The combination of CTLA-4 blockade ipilimumab (Ipi) with VEGF-A blocking antibody bevacizumab (Bev) has demonstrated favorable clinical outcomes in patients with advanced melanoma. Galectin-3 (Gal-3) plays a prominent role in tumor growth, metastasis, angiogenesis, and immune evasion. Here we report that Ipi plus Bev (Ipi-Bev) therapy increased Gal-3 antibody titers by 50% or more in approximately one third of treated patients. Antibody responses to Gal-3 were associated with higher complete and partial responses and better overall survival. Ipi alone also elicited antibody responses to Gal-3 at a frequency comparable to the Ipi-Bev combination. However, an association of elicited antibody responses to Gal-3 with clinical outcomes was not observed in Ipi alone treated patients. In contrast to being neutralized in Ipi-Bev treated patients, circulating VEGF-A increased by 100% or more in a subset of patients after Ipi treatment, with most having progressive disease. Among the Ipi treated patients with therapy-induced Gal-3 antibody increases, circulating VEGF-A was increased in 3 of 6 nonresponders but in none of 4 responders as a result of treatment. Gal-3 antibody responses occurred significantly less frequently (3.2%) in a cohort of patients receiving PD-1 blockade where high pre-treatment serum Gal-3 was associated with reduced OS and response rates. Our findings suggest that anti-CTLA-4 elicited humoral immune responses to Gal-3 in melanoma patients which may contribute to the antitumor effect in the presence of an anti-VEGF-A combination. Furthermore, pre-treatment circulating Gal-3 may potentially have prognostic and predictive value for immune checkpoint therapy.
Keywords: anti-VEGF; antibody responses to galectin-3; galectin-3; immune therapy; melanoma.
Figures

References
-
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Eng J Med. 2011;364:2517–26. - PubMed
-
- Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–72. - PubMed
-
- Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
