D-2-hydroxyglutarate interferes with HIF-1α stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization
- PMID: 29900057
- PMCID: PMC5993507
- DOI: 10.1080/2162402X.2018.1445454
D-2-hydroxyglutarate interferes with HIF-1α stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization
Abstract
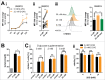
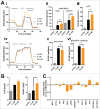
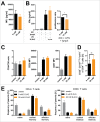
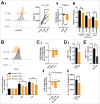
D-2-hydroxyglutarate (D-2HG) is released by various types of malignant cells including acute myeloid leukemia (AML) blasts carrying isocitrate dehydrogenase (IDH) gain-of-function mutations. D-2HG acting as an oncometabolite promotes proliferation, anoikis, and differentiation block of hematopoietic cells in an autocrine fashion. However, prognostic impact of IDH mutations and high D-2HG levels remains controversial and might depend on the overall mutational context. An increasing number of studies focus on the permissive environment created by AML blasts to promote immune evasion. Impact of D-2HG on immune cells remains incompletely understood. Here, we sought out to investigate the effects of D-2HG on T-cells as key mediators of anti-AML immunity. D-2HG was efficiently taken up by T-cells in vitro, which is in line with high 2-HG levels measured in T-cells isolated from AML patients carrying IDH mutations. T-cell activation was slightly impacted by D-2HG. However, D-2HG triggered HIF-1a protein destabilization resulting in metabolic skewing towards oxidative phosphorylation, increased regulatory T-cell (Treg) frequency, and reduced T helper 17 (Th17) polarization. Our data suggest for the first time that D-2HG might contribute to fine tuning of immune responses.
Keywords: 2HG; IDH mutation; Immunosurveillance; Inflammation and cancer; Th17; Tregs; acute myeloid leukemia; immunometabolism; oncometabolite.
Figures

References
-
- Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, et al.. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–34. doi: 10.1016/j.ccr.2010.01.020. PMID:20171147. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
