The possibility of cancer immune editing in gliomas. A critical review
- PMID: 29900059
- PMCID: PMC5993488
- DOI: 10.1080/2162402X.2018.1445458
The possibility of cancer immune editing in gliomas. A critical review
Abstract
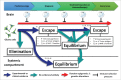
The relationship between anti-tumoral immunity and cancer progression is complex. Recently, immune editing has emerged as a model to explain the interplay between the immune system and the selection of genetic alterations in cancer. In this model, the immune system selects cancer cells that grow as these are fit to escape immune surveillance during tumor development. Gliomas and glioblastoma, the most aggressive and most common of all primary malignant brain tumors are genetically heterogeneous, are relatively less antigenic, and are less responsive to immunotherapy than other cancers. In this review, we provide an overview of the relationship between glioma´s immune suppressive features, anti-tumoral immunity and cancer genomics. In this context, we provide a critical discussion of evidence suggestive of immune editing in this disease and discuss possible alternative explanations for these findings.
Keywords: cancer genomics; cancer immune editing; equilibrium; escape; glioma.
Figures

References
-
- Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7. PMID:14612546. - PubMed
-
- Jacobs JF, Idema AJ, Bol KF, Nierkens S, Grauer OM, Wesseling P, Grotenhuis JA, Hoogerbrugge PM, de Vries IJ, Adema GJ. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009;11(4):394–402. doi:10.1215/15228517-2008-104. PMID:19028999. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
