Residual Peripheral Blood CD26+ Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission
- PMID: 29900128
- PMCID: PMC5988870
- DOI: 10.3389/fonc.2018.00194
Residual Peripheral Blood CD26+ Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission
Abstract
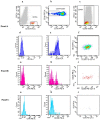
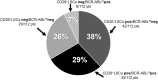
Chronic myeloid leukemia (CML) patients in sustained "deep molecular response" may stop TKI treatment without disease recurrence; however, half of them lose molecular response shortly after TKI withdrawing. Well-defined eligibility criteria to predict a safe discontinuation up-front are still missing. Relapse is probably due to residual quiescent TKI-resistant leukemic stem cells (LSCs) supposedly transcriptionally low/silent and not easily detectable by BCR-ABL1 qRT-PCR. Bone marrow Ph+ CML CD34+/CD38- LSCs were found to specifically co-express CD26 (dipeptidylpeptidase-IV). We explored feasibility of detecting and quantifying CD26+ LSCs by flow cytometry in peripheral blood (PB). Over 400 CML patients (at diagnosis and during/after therapy) entered this cross-sectional study in which CD26 expression was evaluated by a standardized multiparametric flow cytometry analysis on PB CD45+/CD34+/CD38- stem cell population. All 120 CP-CML patients at diagnosis showed measurable PB CD26+ LSCs (median 19.20/μL, range 0.27-698.6). PB CD26+ LSCs were also detectable in 169/236 (71.6%) CP-CML patients in first-line TKI treatment (median 0.014 cells/μL; range 0.0012-0.66) and in 74/112 (66%), additional patients studied on treatment-free remission (TFR) (median 0.015/μL; range 0.006-0.76). Notably, no correlation between BCR-ABL/ABLIS ratio and number of residual LSCs was found both in patients on or off TKIs. This is the first evidence that "circulating" CML LSCs persist in the majority of CML patients in molecular response while on TKI treatment and even after TKI discontinuation. Prospective studies evaluating the dynamics of PB CD26+ LSCs during TKI treatment and the role of a "stem cell response" threshold to achieve and maintain TFR are ongoing.
Keywords: CD26; TKI; chronic myeloid leukemia; flow cytometry; leukemic stem cells; minimal residual disease.
Figures

References
-
- Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol (2010) 11(11):1029–35.10.1016/S1470-2045(10)70233-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

