Structural data of DNA binding and molecular docking studies of dihydropyrimidinone transition metal complexes
- PMID: 29900378
- PMCID: PMC5997584
- DOI: 10.1016/j.dib.2018.04.040
Structural data of DNA binding and molecular docking studies of dihydropyrimidinone transition metal complexes
Abstract
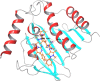
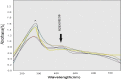
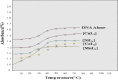
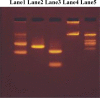
A series of some novel copper complexes derived from Biginelli condensation of DHPHS. The ligand and its transition metal complexes show more antimicrobial activities which was substantiated by molecular docking studies. Transition metal complexes four possess antioxidant properties supported by the DNA-binding, cleavage, and viscosity measurement (Prasad et al., 2011) [1]. The in Silicon DNA binding reveals copper complex is bound to be Minor groove and other manganese, cobalt, nickel complexes are bound to the Major groove portion of DNA through hydrogen bonds and hence copper (II), manganese (II), cobalt (II), nickel (II) complexes are called Minor groove and Major groove binder respectively. The DNA cleavage studies of metal complexes presented more protruding activity in the attendance of H2O2 associated to that in the absence of H2O2. In continuance of our ongoing research on DNA binding and cleavage happenings of transition metal complexes, in this paper we obtainable the synthesis, characterization and DNA cleavage activities.
Keywords: 4-aminoantipyrine; Antioxidant; DHPHs; DNA binding; DNA cleavage; Molecular docking.
Figures



References
-
- Prasad K.S., Shiva Kumar L., Chandran S., Jayalakshmi B., Revanasiddappaa H.D. Diorganotin(IV) complexes of biologically potent 4(3H)-quinazolinone derived Schiff bases: synthesis, spectroscopic characterization, DNA interaction studies and antimicrobial activity. Spectrochim. Acta Part A. 2011;81:276–282. - PubMed
-
- Hitoshi T., Tamao N., Hideyuki A., Manabu, Takayuki M. Preparation and characterization of novel cyclic tetranuclear manganese (III) complexes: mnIII4(X-salmphen)6 (X-salmphenH2=N,N′-di-substituted-salicylidene-1,3-diaminobenzene (X=H, 5-Br) Polyhedron. 1997;16:3787–3794.
-
- Punniyamurthy T., Kalra S.J.S., Iqbal J. Cobalt(II) catalyzed biomimetic oxidation of hydrocarbons in the presence of dioxygen and 2-methylpropanal. Tetrahedron Lett. 1995;36:8497–8500.
LinkOut - more resources
Full Text Sources
Other Literature Sources

