Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson's disease
- PMID: 29900402
- PMCID: PMC5988730
- DOI: 10.1038/s41531-018-0050-8
Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson's disease
Abstract
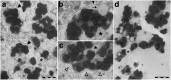
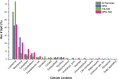
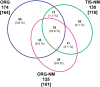
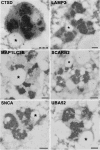
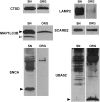
During aging, neuronal organelles filled with neuromelanin (a dark-brown pigment) and lipid bodies accumulate in the brain, particularly in the substantia nigra, a region targeted in Parkinson's disease. We have investigated protein and lipid systems involved in the formation of these organelles and in the synthesis of the neuromelanin of human substantia nigra. Membrane and matrix proteins characteristic of lysosomes were found in neuromelanin-containing organelles at a lower number than in typical lysosomes, indicating a reduced enzymatic activity and likely impaired capacity for lysosomal and autophagosomal fusion. The presence of proteins involved in lipid transport may explain the accumulation of lipid bodies in the organelle and the lipid component in neuromelanin structure. The major lipids observed in lipid bodies of the organelle are dolichols with lower amounts of other lipids. Proteins of aggregation and degradation pathways were present, suggesting a role for accumulation by this organelle when the ubiquitin-proteasome system is inadequate. The presence of proteins associated with aging and storage diseases may reflect impaired autophagic degradation or impaired function of lysosomal enzymes. The identification of typical autophagy proteins and double membranes demonstrates the organelle's autophagic nature and indicates that it has engulfed neuromelanin precursors from the cytosol. Based on these data, it appears that the neuromelanin-containing organelle has a very slow turnover during the life of a neuron and represents an intracellular compartment of final destination for numerous molecules not degraded by other systems.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Duffy P, Tennyson VM. Phase and electron microscopic observations of Lewy bodies and melanin granules in the substantia nigra and locus coeruleus in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1965;24:398–414. doi: 10.1097/00005072-196507000-00003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

