Apical Cell-Cell Adhesions Reconcile Symmetry and Asymmetry in Zebrafish Neurulation
- PMID: 29901027
- PMCID: PMC5994761
- DOI: 10.1016/j.isci.2018.04.007
Apical Cell-Cell Adhesions Reconcile Symmetry and Asymmetry in Zebrafish Neurulation
Abstract
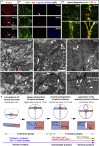
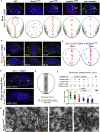
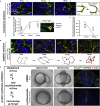
The symmetric tissue and body plans of animals are paradoxically constructed with asymmetric cells. To understand how the yin-yang duality of symmetry and asymmetry are reconciled, we asked whether apical polarity proteins orchestrate the development of the mirror-symmetric zebrafish neural tube by hierarchically modulating apical cell-cell adhesions. We found that apical polarity proteins localize by a pioneer-intermediate-terminal order. Pioneer proteins establish the mirror symmetry of the neural rod by initiating two distinct types of apical adhesions: the parallel apical adhesions (PAAs) cohere cells of parallel orientation and the novel opposing apical adhesions (OAAs) cohere cells of opposing orientation. Subsequently, the intermediate proteins selectively augment the PAAs when the OAAs dissolve by endocytosis. Finally, terminal proteins are required to inflate the neural tube by generating osmotic pressure. Our findings suggest a general mechanism to construct mirror-symmetric tissues: tissue symmetry can be established by organizing asymmetric cells opposingly via adhesions.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures





References
-
- Bagnat M., Cheung I.D., Mostov K.E., Stainier D.Y. Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol. 2007;9:954–960. - PubMed
-
- Barald K.F., Kelley M.W. From placode to polarization: new tunes in inner ear development. Development. 2004;17:4119–4130. - PubMed
-
- Buckley C., Clarke J. Establishing the plane of symmetry for lumen formation and bilateral brain formation in the zebrafish neural rod. Semin. Cell Dev. Biol. 2014;31:100–105. - PubMed
-
- Cadwell C.M., Su W., Kowalczyk A.P. Cadherin tales: regulation of cadherin function by endocytic membrane trafficking. Traffic. 2016;17:1262–1271. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

