Microwave hyperthermia promotes caspase‑3-dependent apoptosis and induces G2/M checkpoint arrest via the ATM pathway in non‑small cell lung cancer cells
- PMID: 29901106
- PMCID: PMC6017221
- DOI: 10.3892/ijo.2018.4439
Microwave hyperthermia promotes caspase‑3-dependent apoptosis and induces G2/M checkpoint arrest via the ATM pathway in non‑small cell lung cancer cells
Abstract
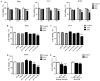
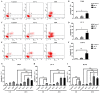
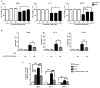
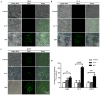
Post-operative microwave (MW) hyperthermia has been applied as an important adjuvant therapy to enhance the efficacy of traditional cancer treatment. A better understanding of the molecular mechanisms of MW hyperthermia may provide guided and further information on clinical hyperthermia treatment. In this study, we examined the effects of MW hyperthermia on non‑small cell lung carcinoma (NSCLC) cells in vitro, as well as the underlying mechanisms. In order to mimic clinical treatment, we developed special MW heating equipment for this study. Various NSCLC cells (H460, PC-9 and H1975) were exposed to hyperthermia treatment using a water bath or MW heating system. The results revealed that MW hyperthermia significantly inhibited cell growth compared with the water bath heating system. Furthermore, MW hyperthermia increased the production of reactive oxygen species (ROS), decreased the levels of mitochondrial membrane potential (MMP) and induced caspase‑3 dependent apoptosis. It also induced G2/M phase arrest through the upregulation of the expression of phosphorylated (p‑) ataxia telangiectasia mutated (ATM), p‑checkpoint kinase 2 (Chk2) and p21, and the downregulation of the expression of cdc25c, cyclin B1 and cdc2. On the whole, the findings of this study indicate that the exposure of NSCLC cells to MW hyperthermia promotes caspase‑3 dependent apoptosis and induces G2/M cell cycle arrest via the ATM pathway. This preclinical study may help to provide laboratory-based evidence for MW hyperthermia treatment in clinical practice.
Figures


Similar articles
-
Ovatodiolide isolated from Anisomeles indica induces cell cycle G2/M arrest and apoptosis via a ROS-dependent ATM/ATR signaling pathways.Eur J Pharmacol. 2018 Jan 15;819:16-29. doi: 10.1016/j.ejphar.2017.09.050. Epub 2017 Oct 3. Eur J Pharmacol. 2018. PMID: 28986085
-
Bortezomib induces G2-M arrest in human colon cancer cells through ROS-inducible phosphorylation of ATM-CHK1.Int J Oncol. 2012 Jul;41(1):76-82. doi: 10.3892/ijo.2012.1448. Epub 2012 Apr 26. Int J Oncol. 2012. PMID: 22552540
-
Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations.Free Radic Biol Med. 2014 Sep;74:1-13. doi: 10.1016/j.freeradbiomed.2014.06.008. Epub 2014 Jun 19. Free Radic Biol Med. 2014. PMID: 24952138
-
Micro-Nanomaterials for Tumor Microwave Hyperthermia: Design, Preparation, and Application.Curr Drug Deliv. 2017;14(3):307-322. doi: 10.2174/1567201813666160108113805. Curr Drug Deliv. 2017. PMID: 26743355 Review.
-
The Complex Roles of DNA Repair Pathways, Inhibitors, Hyperthermia, and Contact Inhibition in Cell Cycle Halts.Mini Rev Med Chem. 2023;23(5):514-529. doi: 10.2174/1389557522666220826141837. Mini Rev Med Chem. 2023. PMID: 36029081 Review.
Cited by
-
Cancer cells resist hyperthermia due to its obstructed activation of caspase 3.Rep Pract Oncol Radiother. 2020 May-Jun;25(3):323-326. doi: 10.1016/j.rpor.2020.02.008. Epub 2020 Feb 26. Rep Pract Oncol Radiother. 2020. PMID: 32194353 Free PMC article. Review.
-
CISD2 promotes lung squamous carcinoma cell migration and invasion via the TGF-β1-induced Smad2/3 signaling pathway.Clin Transl Oncol. 2023 Dec;25(12):3527-3540. doi: 10.1007/s12094-023-03222-5. Epub 2023 May 30. Clin Transl Oncol. 2023. PMID: 37249759
-
Combination Therapy with Cinnamaldehyde and Hyperthermia Induces Apoptosis of A549 Non-Small Cell Lung Carcinoma Cells via Regulation of Reactive Oxygen Species and Mitogen-Activated Protein Kinase Family.Int J Mol Sci. 2020 Aug 28;21(17):6229. doi: 10.3390/ijms21176229. Int J Mol Sci. 2020. PMID: 32872198 Free PMC article.
-
Photothermal treatment-based heat stress regulates function of myeloid-derived suppressor cells.Sci Rep. 2024 Aug 14;14(1):18847. doi: 10.1038/s41598-024-69074-3. Sci Rep. 2024. PMID: 39143087 Free PMC article.
-
CircHIPK3 prevents chondrocyte apoptosis and cartilage degradation by sponging miR-30a-3p and promoting PON2.Cell Prolif. 2022 Sep;55(9):e13285. doi: 10.1111/cpr.13285. Epub 2022 Jun 18. Cell Prolif. 2022. PMID: 35716032 Free PMC article.
References
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. - DOI - PubMed
-
- Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. - DOI - PMC - PubMed
-
- Wu YL, Sequist LV, Tan EH, Geater SL, Orlov S, Zhang L, Lee KH, Tsai CM, Kato T, Barrios CH, et al. Afatinib as first-line treatment of older patients with EGFR mutation-positive non-small-cell lung cancer: Subgroup analyses of the LUX-lung 3, LUX-lung 6, and LUX-lung 7 trials. Clin Lung Cancer. 2018;S1525-7304(18):30051–2. - PubMed
-
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, et al. European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) European Society for Hyperthermic Oncology (ESHO) Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570. doi: 10.1016/S1470-2045(10)70071-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

