Sequence‑dependent effect of sorafenib in combination with natural phenolic compounds on hepatic cancer cells and the possible mechanism of action
- PMID: 29901131
- PMCID: PMC6089756
- DOI: 10.3892/ijmm.2018.3725
Sequence‑dependent effect of sorafenib in combination with natural phenolic compounds on hepatic cancer cells and the possible mechanism of action
Abstract
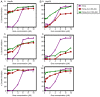
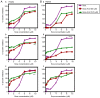
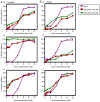
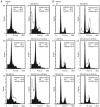
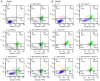
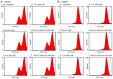
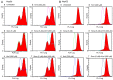
Sorafenib (Nexavar, BAY43‑9006 or Sora) is the first molecular targeted agent that has exhibited significant therapeutic benefits in advanced hepatocellular carcinoma (HCC). However, not all HCC patients respond well to Sora and novel therapeutic strategies to optimize the efficacy of Sora are urgently required. Plant‑based drugs have received increasing attention owing to their excellent chemotherapeutic and chemopreventive activities; they are also well tolerated, non‑toxic, easily available and inexpensive. It is well known that certain biologically active natural products act synergistically with synthetic drugs used in clinical applications. The present study aimed to investigate whether a combination therapy with natural phenolic compounds (NPCs), including curcumin (Cur), quercetin (Que), kaempherol (Kmf) and resveratrol (Rsv), would allow a dose reduction of Sora without concomitant loss of its effectiveness. Furthermore, the possible molecular mechanisms of this synergy were assessed. The hepatic cancer cell lines Hep3b and HepG2 were treated with Sora alone or in combination with NPCs in concomitant, sequential, and inverted sequential regimens. Cell proliferation, cell cycle, apoptosis and expression of proteins associated with the cell cycle and apoptosis were investigated. NPCs markedly potentiated the therapeutic efficacy of Sora in a sequence‑, type‑, NPC dose‑ and cell line‑dependent manner. Concomitant treatment with Sora and Cur [sensitization ratio (SR)=28], Kmf (SR=18) or Que (SR=8) was associated with the highest SRs in Hep3b cells. Rsv markedly potentiated the effect of Sora (SR=17) on Hep3b cells when administered in a reverse sequential manner. By contrast, Rsv and Que did not improve the efficacy of Sora against HepG2 cells, while concomitant treatment with Cur (SR=10) or Kmf (SR=4.01) potentiated the cytotoxicity of Sora. Concomitant treatment with Sora and Cur or Kmf caused S‑phase and G2/M phase arrest of liver cancer cells and markedly induced apoptosis compared with mono‑treatment with Sora, Cur or Kmf. Concomitant treatment with Sora and Cur reduced the protein levels of cyclins A, B2 and D1, phosphorylated retinoblastoma and B‑cell lymphoma (Bcl) extra‑large protein. By contrast, Sora and Cur co‑treatment increased the protein levels of Bcl‑2‑associated X protein, cleaved caspase‑3 and cleaved caspase‑9 in a dose‑dependent manner. In conclusion, concomitant treatment with Sora and Cur or Kmf appears to be a potent and promising therapeutic approach that may control hepatic cancer by triggering cell cycle arrest and apoptosis. Additional studies are required to examine the potential of combined treatment with Sora and NPCs in human hepatic cancer and other solid tumor types in vivo.
Figures






Similar articles
-
Synergy with interferon-lambda 3 and sorafenib suppresses hepatocellular carcinoma proliferation.Biomed Pharmacother. 2017 Apr;88:395-402. doi: 10.1016/j.biopha.2017.01.077. Epub 2017 Jan 22. Biomed Pharmacother. 2017. PMID: 28122304
-
Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib.Oncol Rep. 2018 Sep;40(3):1525-1532. doi: 10.3892/or.2018.6552. Epub 2018 Jul 10. Oncol Rep. 2018. PMID: 30015938
-
Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation.Transplant Proc. 2014 Jan-Feb;46(1):241-4. doi: 10.1016/j.transproceed.2013.10.035. Transplant Proc. 2014. PMID: 24507059
-
The double-edged sword of nutraceuticals: comprehensive review of protective agents and their hidden risks.Front Nutr. 2025 Mar 27;12:1524627. doi: 10.3389/fnut.2025.1524627. eCollection 2025. Front Nutr. 2025. PMID: 40212723 Free PMC article. Review.
-
Natural Compounds Modulating Mitochondrial Functions.Evid Based Complement Alternat Med. 2015;2015:527209. doi: 10.1155/2015/527209. Epub 2015 Jun 18. Evid Based Complement Alternat Med. 2015. PMID: 26167193 Free PMC article. Review.
Cited by
-
Harnessing the Power of Polyphenols: A New Frontier in Disease Prevention and Therapy.Pharmaceuticals (Basel). 2024 May 27;17(6):692. doi: 10.3390/ph17060692. Pharmaceuticals (Basel). 2024. PMID: 38931359 Free PMC article. Review.
-
Antitumor Effects of Quercetin in Hepatocarcinoma In Vitro and In Vivo Models: A Systematic Review.Nutrients. 2019 Nov 25;11(12):2875. doi: 10.3390/nu11122875. Nutrients. 2019. PMID: 31775362 Free PMC article.
-
The pathogenesis of liver cancer and the therapeutic potential of bioactive substances.Front Pharmacol. 2022 Oct 5;13:1029601. doi: 10.3389/fphar.2022.1029601. eCollection 2022. Front Pharmacol. 2022. PMID: 36278230 Free PMC article. Review.
-
Polymeric Nanocarriers for Effective Synergistic Action of Sorafenib Tosylate and Gold-sensitized Gamma Radiation Against HepG2 Cells.Int J Nanomedicine. 2021 Dec 23;16:8309-8321. doi: 10.2147/IJN.S331909. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34992367 Free PMC article.
-
Combining Crocin and Sorafenib Improves Their Tumor-Inhibiting Effects in a Rat Model of Diethylnitrosamine-Induced Cirrhotic-Hepatocellular Carcinoma.Cancers (Basel). 2023 Aug 11;15(16):4063. doi: 10.3390/cancers15164063. Cancers (Basel). 2023. PMID: 37627094 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

