N‑terminal truncated peroxisome proliferator‑activated receptor‑γ coactivator‑1α alleviates phenylephrine‑induced mitochondrial dysfunction and decreases lipid droplet accumulation in neonatal rat cardiomyocytes
- PMID: 29901150
- PMCID: PMC6072228
- DOI: 10.3892/mmr.2018.9158
N‑terminal truncated peroxisome proliferator‑activated receptor‑γ coactivator‑1α alleviates phenylephrine‑induced mitochondrial dysfunction and decreases lipid droplet accumulation in neonatal rat cardiomyocytes
Abstract
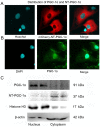
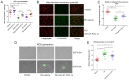
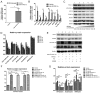
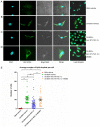
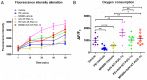
N‑terminal truncated peroxisome proliferator‑activated receptor‑γ coactivator‑1α (NT‑PGC‑1α) is an alternative splice variant of PGC‑1α. NT‑PGC‑1α exhibits stronger anti‑obesity effects in adipose tissue than PGC‑1α; however, NT‑PGC‑1α has not yet been investigated in neonatal rat cardiomyocytes (NRCMs). The present study aimed to investigate the role of NT‑PGC‑1α in mitochondrial fatty acid metabolism and its possible regulatory mechanism in NRCMs. NRCMs were exposed to phenylephrine (PE) or angiotensin II (Ang II) to induce cardiac hypertrophy. Following this, NRCMs were infected with adenovirus expressing NT‑PGC‑1α, and adenosine 5'‑triphsophate (ATP) levels, reactive oxygen species (ROS) generation and mitochondrial membrane potential were subsequently detected. In addition, western blotting, lipid droplet staining and oxygen consumption assays were performed to examine the function of NT‑PGC‑1α in fatty acid metabolism. NT‑PGC‑1α was demonstrated to be primarily expressed in the cytoplasm, which differed from full‑length PGC‑1α, which was predominantly expressed in the nucleus. NT‑PGC‑1α overexpression alleviated mitochondrial function impairment, including ATP generation, ROS production and mitochondrial membrane potential integrity. Furthermore, NT‑PGC‑1α overexpression alleviated the PE‑induced suppression of fatty acid metabolism‑associated protein expression, increased extracellular oxygen consumption and decreased lipid droplet accumulation in NRCMs. Taken together, the present study demonstrated that NT‑PGC‑1α alleviated PE‑induced mitochondrial impairment and decreased lipid droplet accumulation in NRCMs, indicating that NT‑PGC‑1α may have ameliorated mitochondrial energy defects in NRCMs, and may be considered as a potential target for the treatment of heart failure.
Figures
Similar articles
-
Neuropeptide Y damages the integrity of mitochondrial structure and disrupts energy metabolism in cultured neonatal rat cardiomyocytes.Peptides. 2015 Sep;71:162-9. doi: 10.1016/j.peptides.2015.07.001. Epub 2015 Jul 15. Peptides. 2015. PMID: 26188175
-
Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by N‑acetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress.Cell Death Dis. 2018 May 1;9(5):568. doi: 10.1038/s41419-018-0593-y. Cell Death Dis. 2018. PMID: 29752433 Free PMC article.
-
Melatonin alleviates angiotensin-II-induced cardiac hypertrophy via activating MICU1 pathway.Aging (Albany NY). 2020 Nov 26;13(1):493-515. doi: 10.18632/aging.202159. Epub 2020 Nov 26. Aging (Albany NY). 2020. PMID: 33259334 Free PMC article.
-
Peroxisome proliferator‑activated receptor γ coactivator‑1α in heart disease (Review).Mol Med Rep. 2025 Jan;31(1):17. doi: 10.3892/mmr.2024.13382. Epub 2024 Nov 8. Mol Med Rep. 2025. PMID: 39513608 Free PMC article. Review.
-
Mitochondria and Cardiac Hypertrophy.Adv Exp Med Biol. 2017;982:203-226. doi: 10.1007/978-3-319-55330-6_11. Adv Exp Med Biol. 2017. PMID: 28551789 Review.
Cited by
-
Cold-induced expression of a truncated adenylyl cyclase 3 acts as rheostat to brown fat function.Nat Metab. 2024 Jun;6(6):1053-1075. doi: 10.1038/s42255-024-01033-8. Epub 2024 Apr 29. Nat Metab. 2024. PMID: 38684889 Free PMC article.
-
Targeting Adrenergic Receptors in Metabolic Therapies for Heart Failure.Int J Mol Sci. 2021 May 28;22(11):5783. doi: 10.3390/ijms22115783. Int J Mol Sci. 2021. PMID: 34071350 Free PMC article. Review.
-
COX6B1 relieves hypoxia/reoxygenation injury of neonatal rat cardiomyocytes by regulating mitochondrial function.Biotechnol Lett. 2019 Jan;41(1):59-68. doi: 10.1007/s10529-018-2614-4. Epub 2018 Oct 11. Biotechnol Lett. 2019. PMID: 30311029 Free PMC article.
-
PPARα contributes to protection against metabolic and inflammatory derangements associated with acute kidney injury in experimental sepsis.Physiol Rep. 2019 May;7(10):e14078. doi: 10.14814/phy2.14078. Physiol Rep. 2019. PMID: 31102342 Free PMC article.
-
Activation of calcium‑sensing receptor‑mediated autophagy in high glucose‑induced cardiac fibrosis in vitro.Mol Med Rep. 2020 Sep;22(3):2021-2031. doi: 10.3892/mmr.2020.11277. Epub 2020 Jun 26. Mol Med Rep. 2020. PMID: 32705187 Free PMC article.
References
-
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

