Systemic Delivery of AAVB1-GAA Clears Glycogen and Prolongs Survival in a Mouse Model of Pompe Disease
- PMID: 29901418
- PMCID: PMC6343199
- DOI: 10.1089/hum.2018.016
Systemic Delivery of AAVB1-GAA Clears Glycogen and Prolongs Survival in a Mouse Model of Pompe Disease
Abstract
Pompe disease is an autosomal recessive glycogen storage disorder caused by deficiency of the lysosomal enzyme acid alpha-glucosidase (GAA). GAA deficiency results in systemic lysosomal glycogen accumulation and cellular disruption in muscle and the central nervous system (CNS). Adeno-associated virus (AAV) gene therapy is ideal for Pompe disease, since a single systemic injection may correct both muscle and CNS pathologies. Using the Pompe mouse (B6;129-GaaTm1Rabn/J), this study sought to explore if AAVB1, a newly engineered vector with a high affinity for muscle and CNS, reduces systemic weakness and improves survival in adult mice. Three-month-old Gaa-/- animals were injected with either AAVB1 or AAV9 vectors expressing GAA and tissues were harvested 6 months later. Both AAV vectors prolonged survival. AAVB1-treated animals had a robust weight gain compared to the AAV9-treated group. Vector genome levels, GAA enzyme activity, and histological analysis indicated that both vectors transduced the heart efficiently, leading to glycogen clearance, and transduced the diaphragm and CNS at comparable levels. AAVB1-treated mice had higher GAA activity and greater glycogen clearance in the tongue. Finally, AAVB1-treated animals showed improved respiratory function comparable to wild-type animals. In conclusion, AAVB1-GAA offers a promising therapeutic option for the treatment of muscle and CNS in Pompe disease.
Keywords: AAV9; AAVB1; Pompe disease; glycogen; respiratory.
Conflict of interest statement
No competing financial interests exist.
Figures
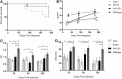




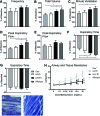
Similar articles
-
Rescue of Advanced Pompe Disease in Mice with Hepatic Expression of Secretable Acid α-Glucosidase.Mol Ther. 2020 Sep 2;28(9):2056-2072. doi: 10.1016/j.ymthe.2020.05.025. Epub 2020 May 30. Mol Ther. 2020. PMID: 32526204 Free PMC article.
-
Gene therapy with secreted acid alpha-glucosidase rescues Pompe disease in a novel mouse model with early-onset spinal cord and respiratory defects.EBioMedicine. 2020 Nov;61:103052. doi: 10.1016/j.ebiom.2020.103052. Epub 2020 Oct 9. EBioMedicine. 2020. PMID: 33039711 Free PMC article.
-
Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice.Mol Ther. 2014 Apr;22(4):702-12. doi: 10.1038/mt.2013.282. Epub 2013 Dec 12. Mol Ther. 2014. PMID: 24336173 Free PMC article.
-
Gene Therapy for Pompe Disease: The Time is now.Hum Gene Ther. 2019 Oct;30(10):1245-1262. doi: 10.1089/hum.2019.109. Epub 2019 Sep 9. Hum Gene Ther. 2019. PMID: 31298581 Review.
-
What's new and what's next for gene therapy in Pompe disease?Expert Opin Biol Ther. 2022 Sep;22(9):1117-1135. doi: 10.1080/14712598.2022.2067476. Epub 2022 Apr 27. Expert Opin Biol Ther. 2022. PMID: 35428407 Free PMC article.
Cited by
-
Glycogen accumulation in smooth muscle of a Pompe disease mouse model.J Smooth Muscle Res. 2021;57(0):8-18. doi: 10.1540/jsmr.57.8. J Smooth Muscle Res. 2021. PMID: 33883348 Free PMC article.
-
Molecular Approaches for the Treatment of Pompe Disease.Mol Neurobiol. 2020 Feb;57(2):1259-1280. doi: 10.1007/s12035-019-01820-5. Epub 2019 Nov 12. Mol Neurobiol. 2020. PMID: 31713816 Review.
-
Current avenues of gene therapy in Pompe disease.Curr Opin Neurol. 2023 Oct 1;36(5):464-473. doi: 10.1097/WCO.0000000000001187. Epub 2023 Jul 19. Curr Opin Neurol. 2023. PMID: 37639402 Free PMC article. Review.
-
Two-Plasmid Packaging System for Recombinant Adeno-Associated Virus.Biores Open Access. 2020 Oct 16;9(1):219-228. doi: 10.1089/biores.2020.0031. eCollection 2020. Biores Open Access. 2020. PMID: 33117614 Free PMC article.
-
Advances in Pompe Disease Treatment: From Enzyme Replacement to Gene Therapy.Mol Diagn Ther. 2024 Nov;28(6):703-719. doi: 10.1007/s40291-024-00733-x. Epub 2024 Aug 12. Mol Diagn Ther. 2024. PMID: 39134822 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

