A Review of Current Concepts of the Etiology and Treatment of Myopia
- PMID: 29901472
- PMCID: PMC6023584
- DOI: 10.1097/ICL.0000000000000499
A Review of Current Concepts of the Etiology and Treatment of Myopia
Abstract
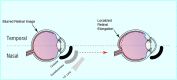
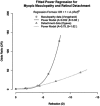
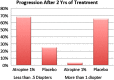
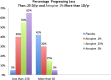
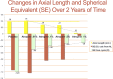
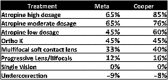
Myopia occurs in more than 50% of the population in many industrialized countries and is expected to increase; complications associated with axial elongation from myopia are the sixth leading cause of blindness. Thus, understanding its etiology, epidemiology, and the results of various treatment regiments may modify current care and result in a reduction in morbidity from progressive myopia. This rapid increase cannot be explained by genetics alone. Current animal and human research demonstrates that myopia development is a result of the interplay between genetic and the environmental factors. The prevalence of myopia is higher in individuals whose both parents are myopic, suggesting that genetic factors are clearly involved in myopia development. At the same time, population studies suggest that development of myopia is associated with education and the amount time spent doing near work; hence, activities increase the exposure to optical blur. Recently, there has been an increase in efforts to slow the progression of myopia because of its relationship to the development of serious pathological conditions such as macular degeneration, retinal detachments, glaucoma, and cataracts. We reviewed meta-analysis and other of current treatments that include: atropine, progressive addition spectacle lenses, orthokeratology, and multifocal contact lenses.
Conflict of interest statement
J. Cooper: Consultant to VTI (Visioneering Technologies, Inc., Alpharetta, GA), Treehouse Eyes, and Magic Leap, the remaining author has no funding or conflicts of interest to disclose.
Figures



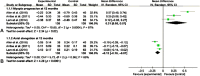


References
-
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012;31:622–660. - PubMed
-
- Holden BA. The Charles F. Prentice award Lecture 2014: A 50-year research journey: Giants and Great Collaborators. Optom Vis Sci 2015;92:741–749. - PubMed
-
- Pararajasegaram R. VISION 2020-the right to sight: From strategies to action. Am J Ophthalmol 1999;128:359–360. - PubMed
-
- Kempen JH, Mitchell P, Lee KE, et al. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol 2004;122:495–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

