Curcumin induces endoplasmic reticulum stress-associated apoptosis in human papillary thyroid carcinoma BCPAP cells via disruption of intracellular calcium homeostasis
- PMID: 29901626
- PMCID: PMC6023948
- DOI: 10.1097/MD.0000000000011095
Curcumin induces endoplasmic reticulum stress-associated apoptosis in human papillary thyroid carcinoma BCPAP cells via disruption of intracellular calcium homeostasis
Abstract
Background: Thyroid cancer is the most common endocrine tumor. Our previous studies have demonstrated that curcumin can induce apoptosis in human papillary thyroid carcinoma BCPAP cells. However, the underlined mechanism has not been clearly elucidated. Endoplasmic reticulum (ER) is a major organelle for synthesis, maturation, and folding proteins as well as a large store for Ca. Overcoming chronically activated ER stress by triggering pro-apoptotic pathways of the unfolded protein response (UPR) is a novel strategy for cancer therapeutics. Our study aimed to uncover the ER stress pathway involved in the apoptosis caused by curcumin.
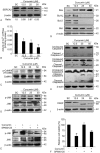
Methods: BCPAP cells were treated with different doses of curcumin (12.5-50 μM). Annexin V/PI double staining was used to determine cell apoptosis. Rhod-2/AM calcium fluorescence probe assay was performed to measure the calcium level of endoplasmic reticulum. Western blot was used to examine the expression of ER stress marker C/EBP homologous protein 10 (CHOP) and glucose-regulated protein 78 (GRP78). X-box binding protein1 (XBP-1) spliced form was examined by reverse transcriptase-polymerase chain reaction (RT-PCR).
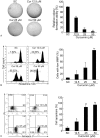
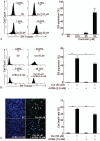
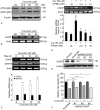
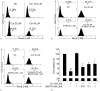
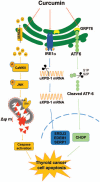
Results: Curcumin significantly inhibited anchorage-independent cell growth and induced apoptosis in BCPAP cells. Curcumin induced ER stress and UPR responses in a dose- and time-dependent manner, and the chemical chaperone 4-phenylbutyrate (4-PBA) partially reversed the antigrowth activity of curcumin. Moreover, curcumin significantly increased inositol-requiring enzyme 1α (IRE1α) phosphorylation and XBP-1 mRNA splicing to induce a subsets of ER chaperones. Increased cleavage of activating transcription factor 6 (ATF6), which enhances expression of its downstream target CHOP was also observed. Furthermore, curcumin induced intracellular Ca influx through inhibition of the sarco-endoplasmic reticulum ATPase 2A (SERCA2) pump. The increased cytosolic Ca then bound to calmodulin to activate calcium/calmodulin-dependent protein kinase II (CaMKII) signaling, leading to mitochondrial apoptosis pathway activation. Ca chelator BAPTA partially reversed curcumin-induced ER stress and growth suppression, confirming the possible involvement of calcium homeostasis disruption in this response.
Conclusions: Curcumin inhibits thyroid cancer cell growth, at least partially, through ER stress-associated apoptosis. Our observations provoked that ER stress activation may be a promising therapeutic target for thyroid cancer treatment.(Figure is included in full-text article.).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress.Apoptosis. 2013 Nov;18(11):1391-1402. doi: 10.1007/s10495-013-0871-1. Apoptosis. 2013. PMID: 23881281
-
Tributyltin-induced endoplasmic reticulum stress and its Ca(2+)-mediated mechanism.Toxicol Appl Pharmacol. 2013 Oct 1;272(1):137-46. doi: 10.1016/j.taap.2013.05.026. Epub 2013 Jun 3. Toxicol Appl Pharmacol. 2013. PMID: 23743301
-
A review on endoplasmic reticulum-dependent anti-breast cancer activity of herbal drugs: possible challenges and opportunities.J Drug Target. 2025 Feb;33(2):206-231. doi: 10.1080/1061186X.2024.2417189. Epub 2024 Oct 24. J Drug Target. 2025. PMID: 39404107 Review.
-
ER stress-induced cell death mechanisms.Biochim Biophys Acta. 2013 Dec;1833(12):3460-3470. doi: 10.1016/j.bbamcr.2013.06.028. Epub 2013 Jul 10. Biochim Biophys Acta. 2013. PMID: 23850759 Free PMC article. Review.
Cited by
-
Propofol produces neurotoxicity by inducing mitochondrial apoptosis.Exp Ther Med. 2022 Aug 19;24(4):630. doi: 10.3892/etm.2022.11567. eCollection 2022 Oct. Exp Ther Med. 2022. PMID: 36160898 Free PMC article.
-
Curcumin Inhibits Papillary Thyroid Cancer Cell Proliferation by Regulating lncRNA LINC00691.Anal Cell Pathol (Amst). 2022 Feb 26;2022:5946670. doi: 10.1155/2022/5946670. eCollection 2022. Anal Cell Pathol (Amst). 2022. PMID: 35256924 Free PMC article.
-
Endoplasmic reticulum homeostasis: a potential target for diabetic nephropathy.Front Endocrinol (Lausanne). 2023 Jun 13;14:1182848. doi: 10.3389/fendo.2023.1182848. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37383398 Free PMC article. Review.
-
Curcumin-Loaded Maltodextrin-Based Proniosomes Potentially Effective against Gemcitabine-Resistant Cholangiocarcinoma.ACS Appl Bio Mater. 2025 Jan 20;8(1):913-930. doi: 10.1021/acsabm.4c01832. Epub 2025 Jan 7. ACS Appl Bio Mater. 2025. PMID: 39772434 Free PMC article.
-
Zyflamend induces apoptosis in pancreatic cancer cells via modulation of the JNK pathway.Cell Commun Signal. 2020 Aug 14;18(1):126. doi: 10.1186/s12964-020-00609-7. Cell Commun Signal. 2020. PMID: 32795297 Free PMC article.
References
-
- Carneiro RM, Carneiro BA, Agulnik M, et al. Targeted therapies in advanced differentiated thyroid cancer. Cancer Treat Rev 2015;41:690–8. - PubMed
-
- Heger M, van Golen RF, Broekgaarden M, et al. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev 2014;66:222–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

