Timeliness of notification systems for infectious diseases: A systematic literature review
- PMID: 29902216
- PMCID: PMC6002046
- DOI: 10.1371/journal.pone.0198845
Timeliness of notification systems for infectious diseases: A systematic literature review
Abstract
Introduction: Timely notification of infectious diseases is crucial for prompt response by public health services. Adequate notification systems facilitate timely notification. A systematic literature review was performed to assess outcomes of studies on notification timeliness and to determine which aspects of notification systems are associated with timely notification.
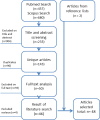
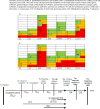
Methodology: Articles reviewing timeliness of notifications published between 2000 and 2017 were searched in Pubmed and Scopus. Using a standardized notification chain, timeliness of reporting system for each article was defined as either sufficient (≥ 80% notifications in time), partly sufficient (≥ 50-80%), or insufficient (< 50%) according to the article's predefined timeframe, a standardized timeframe for all articles, and a disease specific timeframe. Electronic notification systems were compared with conventional methods (postal mail, fax, telephone, email) and mobile phone reporting.
Results: 48 articles were identified. In almost one third of the studies with a predefined timeframe (39), timeliness of notification systems was either sufficient or insufficient (11/39, 28% and 12/39, 31% resp.). Applying the standardized timeframe (45 studies) revealed similar outcomes (13/45, 29%, sufficient notification timeframe, vs 15/45, 33%, insufficient). The disease specific timeframe was not met by any study. Systems involving reporting by laboratories most often complied sufficiently with predefined or standardized timeframes. Outcomes were not related to electronic, conventional notification systems or mobile phone reporting. Electronic systems were faster in comparative studies (10/13); this hardly resulted in sufficient timeliness, neither according to predefined nor to standardized timeframes.
Conclusion: A minority of notification systems meets either predefined, standardized or disease specific timeframes. Systems including laboratory reporting are associated with timely notification. Electronic systems reduce reporting delay, but implementation needs considerable effort to comply with notification timeframes. During outbreak threats, patient, doctors and laboratory testing delays need to be reduced to achieve timely detection and notification. Public health authorities should incorporate procedures for this in their preparedness plans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Gibbons CL, Mangen MJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, et al. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014;14:147 doi: 10.1186/1471-2458-14-147 ; PubMed Central PMCID: PMCPMC4015559. - DOI - PMC - PubMed
-
- German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50(RR-13):1–35; quiz CE1-7. . - PubMed
-
- Jajosky RA, Groseclose SL. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health. 2004;4:29 doi: 10.1186/1471-2458-4-29 . - DOI - PMC - PubMed
-
- Bonacic Marinovic A, Swaan C, van Steenbergen J, Kretzschmar M. Quantifying reporting timeliness to improve outbreak control. Emerg Infect Dis. 2015;21(2):209–16. doi: 10.3201/eid2102.130504 ; PubMed Central PMCID: PMCPMC4313625. - DOI - PMC - PubMed
-
- WHO. International Health Regulations 2005. http://apps.who.int/iris/bitstream/10665/246107/1/9789241580496-eng.pdf?...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

