The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano-TiO2 surfaces
- PMID: 29902240
- PMCID: PMC6002089
- DOI: 10.1371/journal.pone.0199046
The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano-TiO2 surfaces
Abstract
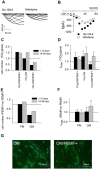
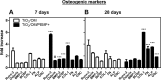
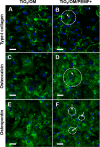
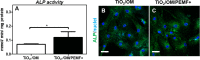
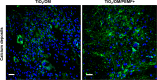
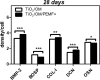
Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) are considered a great promise in the repair and regeneration of bone. Considerable efforts have been oriented towards uncovering the best strategy to promote stem cells osteogenic differentiation. In previous studies, hBM-MSCs exposed to physical stimuli such as pulsed electromagnetic fields (PEMFs) or directly seeded on nanostructured titanium surfaces (TiO2) were shown to improve their differentiation to osteoblasts in osteogenic condition. In the present study, the effect of a daily PEMF-exposure on osteogenic differentiation of hBM-MSCs seeded onto nanostructured TiO2 (with clusters under 100 nm of dimension) was investigated. TiO2-seeded cells were exposed to PEMF (magnetic field intensity: 2 mT; intensity of induced electric field: 5 mV; frequency: 75 Hz) and examined in terms of cell physiology modifications and osteogenic differentiation. Results showed that PEMF exposure affected TiO2-seeded cells osteogenesis by interfering with selective calcium-related osteogenic pathways, and greatly enhanced hBM-MSCs osteogenic features such as the expression of early/late osteogenic genes and protein production (e.g., ALP, COL-I, osteocalcin and osteopontin) and ALP activity. Finally, PEMF-treated cells resulted to secrete into conditioned media higher amounts of BMP-2, DCN and COL-I than untreated cell cultures. These findings confirm once more the osteoinductive potential of PEMF, suggesting that its combination with TiO2 nanostructured surface might be a great option in bone tissue engineering applications.
Conflict of interest statement
The authors received funding from the Compagnia di San Paolo. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
References
-
- Viganò M, Sansone V, d’Agostino MC, Romeo P, Perucca Orfei C, de Girolamo L. Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders. J Orthop Surg Res. 2016;11: 163 doi: 10.1186/s13018-016-0496-5 - DOI - PMC - PubMed
-
- Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7: 131 doi: 10.1186/s13287-016-0394-0 - DOI - PMC - PubMed
-
- Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005;1: 211–22. doi: 10.1016/j.actbio.2004.11.009 - DOI - PubMed
-
- Chen W, Shao Y, Li X, Zhao G, Fu J. Nanotopographical Surfaces for Stem Cell Fate Control: Engineering Mechanobiology from the Bottom. Nano Today. 2014;9: 759–784. doi: 10.1016/j.nantod.2014.12.002 - DOI - PMC - PubMed
-
- Mangano C, De Rosa A, Desiderio V, d’Aquino R, Piattelli A, De Francesco F, et al. The osteoblastic differentiation of dental pulp stem cells and bone formation on different titanium surface textures. Biomaterials. 2010;31: 3543–3551. doi: 10.1016/j.biomaterials.2010.01.056 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

