Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation
- PMID: 29902862
- PMCID: PMC6319543
- DOI: 10.4062/biomolther.2017.260
Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation
Abstract
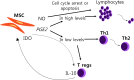
Mesenchymal stem cells are classified as multipotent stem cells, due to their capability to transdifferentiate into various lineages that develop from mesoderm. Their popular appeal as cell-based therapy was initially based on the idea of their ability to restore tissue because of their differentiation potential in vitro; however, the lack of evidence of their differentiation to target cells in vivo led researchers to focus on their secreted trophic factors and their role as potential powerhouses on regulation of factors under different immunological environments and recover homeostasis. To date there are more than 800 clinical trials on humans related to MSCs as therapy, not to mention that in animals is actively being applied as therapeutic resource, though it has not been officially approved as one. But just as how results from clinical trials are important, so is to reveal the biological mechanisms involved on how these cells exert their healing properties to further enhance the application of MSCs on potential patients. In this review, we describe characteristics of MSCs, evaluate their benefits as tissue regenerative therapy and combination therapy, as well as their immunological properties, activation of MSCs that dictate their secreted factors, interactions with other immune cells, such as T cells and possible mechanisms and pathways involved in these interactions.
Keywords: Immunomodulation; Mesenchymal stem cells; Prostaglandin E2; Regenerative medicine; T regulators; Toll-like receptor.
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures


References
-
- Abouelkheir M, Eltantawy DA, Saad M.-A, Abdelrahman KM, Sobh M.-A, Lotfy A, Sobh MA. Mesenchymal stem cells versus their conditioned medium in the treatment of cisplatin-induced acute kidney injury: evaluation of efficacy and cellular side effects. Int. J. Clin. Exp. Med. 2016;9:23222–23234.
-
- Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, Aguila-Diaz C, Fernandez A, Figueroa FE, Khoury M. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2015;6:32. doi: 10.1186/s13287-015-0013-5. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

