The neuropathology of the adult cerebellum
- PMID: 29903436
- PMCID: PMC6279249
- DOI: 10.1016/B978-0-444-63956-1.00008-4
The neuropathology of the adult cerebellum
Abstract
This chapter summarizes the neuropathologic features of nonneoplastic disorders of the adult cerebellum. Gait ataxia and extremity dysmetria are clinical manifestations of diseases that interrupt the complex cerebellar circuitry between the neurons of the cerebellar cortex, the cerebellar nuclei (especially the dentate nuclei), and the inferior olivary nuclei. The cerebellum is a prominent target of several sporadic and hereditary neurodegenerative diseases, including multiple system atrophy, spinocerebellar ataxia, and Friedreich ataxia. Purkinje cells display selective vulnerability to hypoxia but a surprising resistance to hypoglycemia. A classic toxin that damages the cerebellar cortex is methylmercury, but the most common injurious agent to Purkinje cells is ethanol. Many drugs cause ataxia, but doubts continue about phenytoin. Ischemic lesions of the cerebellum due to arterial thrombosis or embolism cause a spectrum of symptoms and signs, depending on the territory involved. Large hemorrhages have an unfavorable prognosis because they displace critical brainstem structures or penetrate into the fourth ventricle. Fungal infections and toxoplasmosis of the cerebellum, and cerebellar progressive multifocal leukoencephalopathy, have become rarer because of improved control of the acquired immunodeficiency syndrome. Ataxia is a prominent feature of prion disease. Adult-onset Niemann-Pick type C1 disease and Kufs disease may have a predominantly ataxic clinical phenotype. The adult cerebellum is also vulnerable to several leukodystrophies. A rare but widely recognized complication of cancer is paraneoplastic cerebellar degeneration.
Keywords: Friedreich ataxia; cerebellar circuitry; hypoglycemia; hypoxia; infections; leukodystrophy; lipidoses; multiple sclerosis; multiple system atrophy; paraneoplastic cerebellar degeneration; spinocerebellar ataxia; stroke; toxicity.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures






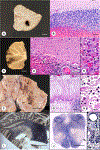
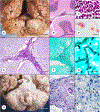
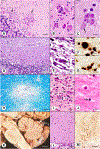



References
-
- Alturkustani M, Sharma M, Hammond R et al. (2013). Adult-onset leukodystrophy: review of 3 clinicopathologic phe-notypes and a proposed classification. J Neuropathol Exp Neurol 72: 1090–1103. - PubMed
-
- Ambrosi G, Flace P, Lorusso L et al. (2007). Non-traditional large neurons in the granular layer of the cerebellar cortex. Eur J Histochem 51 (Suppl 1): 59–64. - PubMed
-
- Antinori A, Larussa D, Cingolani A et al. (2004). Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis 39: 1681–1691. - PubMed
-
- Auer RN, Benveniste H (1997). Hypoxia and related conditions. In: Graham DI, Lantos PL (Eds.), Greenfield’s neuropathology Oxford University Press, New York, pp. 263–314.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

