Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction
- PMID: 29903732
- PMCID: PMC6023725
- DOI: 10.1161/ATVBAHA.118.311156
Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction
Abstract
Objective: Use of alternative tobacco products including electronic cigarettes is rapidly rising. The wide variety of flavored tobacco products available is of great appeal to smokers and youth. The flavorings added to tobacco products have been deemed safe for ingestion, but the cardiovascular health effects are unknown. The purpose of this study was to examine the effect of 9 flavors on vascular endothelial cell function.
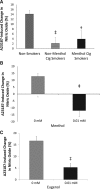
Approach and results: Freshly isolated endothelial cells from participants who use nonmenthol- or menthol-flavored tobacco cigarettes showed impaired A23187-stimulated nitric oxide production compared with endothelial cells from nonsmoking participants. Treatment of endothelial cells isolated from nonsmoking participants with either menthol (0.01 mmol/L) or eugenol (0.01 mmol/L) decreased A23187-stimulated nitric oxide production. To further evaluate the effects of flavoring compounds on endothelial cell phenotype, commercially available human aortic endothelial cells were incubated with vanillin, menthol, cinnamaldehyde, eugenol, dimethylpyrazine, diacetyl, isoamyl acetate, eucalyptol, and acetylpyrazine (0.1-100 mmol/L) for 90 minutes. Cell death, reactive oxygen species production, expression of the proinflammatory marker IL-6 (interleukin-6), and nitric oxide production were measured. Cell death and reactive oxygen species production were induced only at high concentrations unlikely to be achieved in vivo. Lower concentrations of selected flavors (vanillin, menthol, cinnamaldehyde, eugenol, and acetylpyridine) induced both inflammation and impaired A23187-stimulated nitric oxide production consistent with endothelial dysfunction.
Conclusions: Our data suggest that short-term exposure of endothelial cells to flavoring compounds used in tobacco products have adverse effects on endothelial cell phenotype that may have relevance to cardiovascular toxicity.
Keywords: endothelial cells; eugenol; inflammation; nitric oxide; tobacco.
© 2018 American Heart Association, Inc.
Figures





References
-
- Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes—will England reframe the debate? N Engl J Med. 2016;374:1301–1303. doi: 10.1056/NEJMp1601154. - PubMed
-
- Fairchild AL, Lee JS, Bayer R, Curran J. E-cigarettes and the harm-reduction continuum. N Engl J Med. 2018;378:216–219. doi: 10.1056/NEJMp1711991. - PubMed
-
- Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. - PubMed
-
- Neff LJ, Arrazola RA, Caraballo RS, Corey CG, Cox S, King BA, Choiniere CJ, Husten CG. Frequency of tobacco use among middle and high school students—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1061–1065. doi: 10.15585/mmwr.mm6438a1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

